Inter- vs. Intramolecular Hydrogen Bond Patterns and Proton Dynamics in Nitrophthalic Acid Associates
- PMID: 33066679
- PMCID: PMC7587347
- DOI: 10.3390/molecules25204720
Inter- vs. Intramolecular Hydrogen Bond Patterns and Proton Dynamics in Nitrophthalic Acid Associates
Abstract
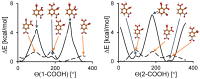
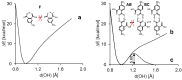
Noncovalent interactions are among the main tools of molecular engineering. Rational molecular design requires knowledge about a result of interplay between given structural moieties within a given phase state. We herein report a study of intra- and intermolecular interactions of 3-nitrophthalic and 4-nitrophthalic acids in the gas, liquid, and solid phases. A combination of the Infrared, Raman, Nuclear Magnetic Resonance, and Incoherent Inelastic Neutron Scattering spectroscopies and the Car-Parrinello Molecular Dynamics and Density Functional Theory calculations was used. This integrated approach made it possible to assess the balance of repulsive and attractive intramolecular interactions between adjacent carboxyl groups as well as to study the dependence of this balance on steric confinement and the effect of this balance on intermolecular interactions of the carboxyl groups.
Keywords: CPMD; DFT; IINS; IR; NMR; Raman; carboxyl group; proton dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
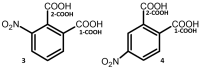
Similar articles
-
Inter- vs. Intra-Molecular Hydrogen Bond in Complexes of Nitrophthalic Acids with Pyridine.Int J Mol Sci. 2023 Mar 9;24(6):5248. doi: 10.3390/ijms24065248. Int J Mol Sci. 2023. PMID: 36982321 Free PMC article.
-
Very Strong Hydrogen Bond in Nitrophthalic Cocrystals.Molecules. 2024 Jul 29;29(15):3565. doi: 10.3390/molecules29153565. Molecules. 2024. PMID: 39124970 Free PMC article.
-
Vibrations and reorientations of H2O molecules in [Sr(H2O)6]Cl2 studied by Raman light scattering, incoherent inelastic neutron scattering and proton magnetic resonance.Spectrochim Acta A Mol Biomol Spectrosc. 2014 Apr 24;124:429-40. doi: 10.1016/j.saa.2014.01.054. Epub 2014 Jan 21. Spectrochim Acta A Mol Biomol Spectrosc. 2014. PMID: 24508882
-
Ultrafast chemistry: using time-resolved vibrational spectroscopy for interrogation of structural dynamics.Annu Rev Phys Chem. 2005;56:337-67. doi: 10.1146/annurev.physchem.56.092503.141314. Annu Rev Phys Chem. 2005. PMID: 15796704 Review.
-
Intramolecular Hydrogen Bonding Involving Organic Fluorine: NMR Investigations Corroborated by DFT-Based Theoretical Calculations.Molecules. 2017 Mar 7;22(3):423. doi: 10.3390/molecules22030423. Molecules. 2017. PMID: 28272370 Free PMC article. Review.
Cited by
-
Inter- vs. Intra-Molecular Hydrogen Bond in Complexes of Nitrophthalic Acids with Pyridine.Int J Mol Sci. 2023 Mar 9;24(6):5248. doi: 10.3390/ijms24065248. Int J Mol Sci. 2023. PMID: 36982321 Free PMC article.
-
3D-printed liquid metal polymer composites as NIR-responsive 4D printing soft robot.Nat Commun. 2023 Nov 28;14(1):7815. doi: 10.1038/s41467-023-43667-4. Nat Commun. 2023. PMID: 38016940 Free PMC article.
-
Structural Studies of Monounsaturated and ω-3 Polyunsaturated Free Fatty Acids in Solution with the Combined Use οf NMR and DFT Calculations-Comparison with the Liquid State.Molecules. 2023 Aug 20;28(16):6144. doi: 10.3390/molecules28166144. Molecules. 2023. PMID: 37630396 Free PMC article.
-
Spectroscopic Identification of Hydrogen Bond Vibrations and Quasi-Isostructural Polymorphism in N-Salicylideneaniline.Molecules. 2021 Aug 20;26(16):5043. doi: 10.3390/molecules26165043. Molecules. 2021. PMID: 34443632 Free PMC article.
-
Modeling of Solute-Solvent Interactions Using an External Electric Field-From Tautomeric Equilibrium in Nonpolar Solvents to the Dissociation of Alkali Metal Halides.Molecules. 2021 Feb 26;26(5):1283. doi: 10.3390/molecules26051283. Molecules. 2021. PMID: 33652943 Free PMC article.
References
-
- Shenderovich I.G., Limbach H.-H., Smirnov S.N., Tolstoy P.M., Denisov G.S., Golubev N.S. H/D isotope effects on the low-temperature NMR parameters and hydrogen bond geometries of (FH)2F− and (FH)3F− dissolved in CDF3/CDF2Cl. Phys. Chem. Chem. Phys. 2002;4:5488–5497. doi: 10.1039/B206323A. - DOI
-
- Mauder D., Akcakayiran D., Lesnichin S.B., Findenegg G.H., Shenderovich I.G. Acidity of Sulfonic and Phosphonic Acid-Functionalized SBA-15 under Almost Water-Free Conditions. J. Phys. Chem. C. 2009;113:19185–19192. doi: 10.1021/jp907058y. - DOI
-
- Melikova S.M., Voronin A.P., Panek J., Frolov N.E., Shishkina A.V., Rykounov A.A., Tretyakov P.Y., Vener M.V. Interplay of pi-stacking and inter-stacking interactions in two-component crystals of neutral closed-shell aromatic compounds: Periodic DFT study. RSC Adv. 2020;10:27899–27910. doi: 10.1039/D0RA04799F. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

