Using the Thickness Map from Macular Ganglion Cell Analysis to Differentiate Retinal Vein Occlusion from Glaucoma
- PMID: 33066685
- PMCID: PMC7602489
- DOI: 10.3390/jcm9103294
Using the Thickness Map from Macular Ganglion Cell Analysis to Differentiate Retinal Vein Occlusion from Glaucoma
Abstract
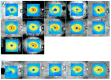
Purpose: We hypothesized that the thickness map from macular ganglion cell analysis (GCA) acquired from spectral-domain optical coherence tomography can be used to differentiate retinal vein occlusion (RVO) from glaucoma.
Methods: In this retrospective case control study, 37 patients with resolved RVO and 74 patients with primary open-angle glaucoma (POAG) were enrolled. Two independent examiners diagnosed patients with RVO or POAG based on the topographic pattern in the GCA thickness map. Inter-observer agreement for a decision between RVO and POAG was assessed using kappa statistics. Diagnostic specificity and accuracy were calculated.
Results: Inter-observer agreement was good, with a kappa value of 0.765 (95% confidence interval, 0.634-0.896, p < 0.001). The diagnostic specificity of RVO from POAG using the GCA thickness map was 93.2% and diagnosis accuracy was 80.4%.
Conclusions: An irregular GCA thickness map represents a simple and convenient differential diagnostic clue to distinguish RVO from POAG.
Keywords: ganglion cell-inner plexiform layer; optical coherence tomography; primary open-angle glaucoma; retinal vein occlusion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Aghi M.K., Chen C.C., Fleseriu M., Newman S.A., Lucas J.W., Kuo J.S., Barkhoudarian G., Farrell C.J., Sheehan J., Ziu M. Congress of neurological surgeons systematic review and evidence-based guidelines on the management of patients with nonfunctioning pituitary adenomas: Executive summary. Neurosurgery. 2016;79:521–523. doi: 10.1227/NEU.0000000000001386. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

