SARS-CoV-2 detection in the lower respiratory tract of invasively ventilated ARDS patients
- PMID: 33066801
- PMCID: PMC7562762
- DOI: 10.1186/s13054-020-03323-5
SARS-CoV-2 detection in the lower respiratory tract of invasively ventilated ARDS patients
Abstract
Background: Data on SARS-CoV-2 load in lower respiratory tract (LRT) are scarce. Our objectives were to describe the viral shedding and the viral load in LRT and to determine their association with mortality in critically ill COVID-19 patients.
Methods: We conducted a binational study merging prospectively collected data from two COVID-19 reference centers in France and Switzerland. First, we described the viral shedding duration (i.e., time to negativity) in LRT samples. Second, we analyzed viral load in LRT samples. Third, we assessed the association between viral presence in LRT and mortality using mixed-effect logistic models for clustered data adjusting for the time between symptoms' onset and date of sampling.
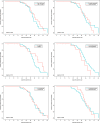
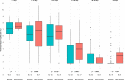
Results: From March to May 2020, 267 LRT samples were performed in 90 patients from both centers. The median time to negativity was 29 (IQR 23; 34) days. Prolonged viral shedding was not associated with age, gender, cardiac comorbidities, diabetes, immunosuppression, corticosteroids use, or antiviral therapy. The LRT viral load tended to be higher in non-survivors. This difference was statistically significant after adjusting for the time interval between onset of symptoms and date of sampling (OR 3.78, 95% CI 1.13-12.64, p = 0.03).
Conclusions: The viral shedding in LRT lasted almost 30 days in median in critically ill patients, and the viral load in the LRT was associated with the 6-week mortality.
Trial registration: ClinicalTrials.gov NCT04262921.
Keywords: COVID-19; ICU; Lower respiratory tract; Mortality; SARS-CoV-2; Viral load; Viral shedding.
Conflict of interest statement
The authors have disclosed that they do not have conflict of interest. JFT received fees for lectures to 3M, MSD, Pfizer, and Biomerieux. JFT received research grants from Astellas, 3M, MSD, and Pfizer. JFT participated to advisory boards of 3M, MSD, Bayer Pharma, Nabriva, and Pfizer. BV reports grants, personal fees for lectures, and travel accommodations from Qiagen. BV received personal fees for lecture and travel accommodation support from BioMérieux. BV received personal fees for lectures from Hologic and Gilead.
Figures
References
-
- Faust JS, Del Rio C. Assessment of deaths from COVID-19 and from seasonal influenza. JAMA Intern Med. 2020;180(8):1045–46. 10.1001/jamainternmed.2020.2306. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

