COVID-19 Serology at Population Scale: SARS-CoV-2-Specific Antibody Responses in Saliva
- PMID: 33067270
- PMCID: PMC7771435
- DOI: 10.1128/JCM.02204-20
COVID-19 Serology at Population Scale: SARS-CoV-2-Specific Antibody Responses in Saliva
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of an ongoing pandemic that has infected over 36 million and killed over 1 million people. Informed implementation of government public health policies depends on accurate data on SARS-CoV-2 immunity at a population scale. We hypothesized that detection of SARS-CoV-2 salivary antibodies could serve as a noninvasive alternative to serological testing for monitoring of SARS-CoV-2 infection and seropositivity at a population scale. We developed a multiplex SARS-CoV-2 antibody immunoassay based on Luminex technology that comprised 12 CoV antigens, mostly derived from SARS-CoV-2 nucleocapsid (N) and spike (S). Saliva and sera collected from confirmed coronavirus disease 2019 (COVID-19) cases and from the pre-COVID-19 era were tested for IgG, IgA, and IgM to the antigen panel. Matched saliva and serum IgG responses (n = 28) were significantly correlated. The salivary anti-N IgG response resulted in the highest sensitivity (100%), exhibiting a positive response in 24/24 reverse transcription-PCR (RT-PCR)-confirmed COVID-19 cases sampled at >14 days post-symptom onset (DPSO), whereas the salivary anti-receptor binding domain (RBD) IgG response yielded 100% specificity. Temporal kinetics of IgG in saliva were consistent with those observed in blood and indicated that most individuals seroconvert at around 10 DPSO. Algorithms employing a combination of the IgG responses to N and S antigens result in high diagnostic accuracy (100%) by as early as 10 DPSO. These results support the use of saliva-based antibody testing as a noninvasive and scalable alternative to blood-based antibody testing.
Keywords: COVID-19; SARS-CoV-2; antibody test; diagnostics; immunoserology; multiplex; oral fluid; saliva; serology.
Copyright © 2020 American Society for Microbiology.
Figures

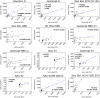

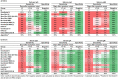
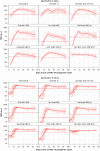
Update of
-
COVID-19 serology at population scale: SARS-CoV-2-specific antibody responses in saliva.medRxiv [Preprint]. 2020 May 26:2020.05.24.20112300. doi: 10.1101/2020.05.24.20112300. medRxiv. 2020. Update in: J Clin Microbiol. 2020 Dec 17;59(1):e02204-20. doi: 10.1128/JCM.02204-20. PMID: 32511537 Free PMC article. Updated. Preprint.
References
-
- Gronvall G, Connel N, Kobokovich A, West R, Warmbrod K, Shearer M, Mullen L, Inglesby T. 2020. Developing a national strategy for serology (antibody testing) in the United States. John Hopkins Bloomberg School of Public Health, Baltimore, MD.
-
- Dyal JW, Grant MP, Broadwater K, Bjork A, Waltenburg MA, Gibbins JD, Hale C, Silver M, Fischer M, Steinberg J, Basler CA, Jacobs JR, Kennedy ED, Tomasi S, Trout D, Hornsby-Myers J, Oussayef NL, Delaney LJ, Patel K, Shetty V, Kline KE, Schroeder B, Herlihy RK, House J, Jervis R, Clayton JL, Ortbahn D, Austin C, Berl E, Moore Z, Buss BF, Stover D, Westergaard R, Pray I, DeBolt M, Person A, Gabel J, Kittle TS, Hendren P, Rhea C, Holsinger C, Dunn J, Turabelidze G, Ahmed FS, deFijter S, Pedati CS, Rattay K, Smith EE, Luna-Pinto C, Cooley LA, et al. . 2020. COVID-19 among workers in meat and poultry processing facilities—19 states, April 2020. MMWR Morb Mortal Wkly Rep 69:557–561. doi:10.15585/mmwr.mm6918e3. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

