Management of a patient with mantle cell lymphoma who developed severe neurotoxicity after chimeric antigen receptor T-cell therapy in ZUMA-2
- PMID: 33067318
- PMCID: PMC7570235
- DOI: 10.1136/jitc-2020-001114
Management of a patient with mantle cell lymphoma who developed severe neurotoxicity after chimeric antigen receptor T-cell therapy in ZUMA-2
Abstract
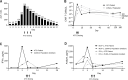
Cerebral edema following chimeric antigen receptor (CAR) T-cell therapy can be fatal. ZUMA-2 is a pivotal phase 2, multicenter study evaluating KTE-X19, an autologous anti-CD19 CAR T-cell therapy, in relapsed/refractory mantle cell lymphoma. We describe a 65-year-old patient in ZUMA-2 who developed cerebral edema following CAR T-cell therapy and had complete recovery after multimodality clinical intervention including rabbit antithymocyte globulin (ATG). Biomarker results show early and robust CAR T-cell expansion and related induction of inflammatory cytokines, followed by rapid declines in CAR T-cell and proinflammatory cytokine levels after ATG administration. This clinical profile highlights a potential relevance of ATG in treating severe CAR T-cell-related neurotoxicity.
Trial registration: ClinicalTrials.gov NCT02601313.
Keywords: case reports; chimeric antigen.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MW: honoraria from Pharmacyclics, Janssen, AstraZeneca, OMI, and Targeted Oncology; consultancy or advisory role for Pharmacyclics, Celgene, Janssen, AstraZeneca, MoreHealth, Pulse Biosciences, Nobel Insights, and Guidpoint Global; research funding from Pharmacyclics, Janssen, Novartis, Juno, Celgene, Loxo Oncology, VelosBio, and Verastem; expert testimony for AstraZeneca; and travel support from Janssen, Pharmacyclics, Celgene, and OMI. TLC: honoraria from Kite, a Gilead Company; consultancy or advisory role for Kite, a Gilead Company. AH: employment at M.D. Anderson; stock or other ownership in Caris; consultancy or advisory role for Caris and WCG Oncology; research funding from Merck; patents, royalties, or other intellectual property from Celldex. LZ and AVR: employment with Gilead Sciences. JMR: employment with Kite, A Gilead Company; stock or other ownership in Gilead Sciences.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
