Astrocytes in the Ventrolateral Preoptic Area Promote Sleep
- PMID: 33067363
- PMCID: PMC7673007
- DOI: 10.1523/JNEUROSCI.1486-20.2020
Astrocytes in the Ventrolateral Preoptic Area Promote Sleep
Abstract
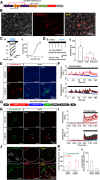
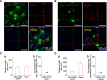
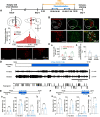
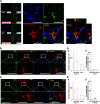
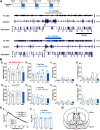
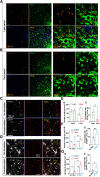
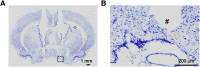
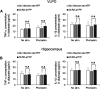
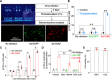
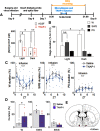
Although ventrolateral preoptic (VLPO) nucleus is regarded as a center for sleep promotion, the exact mechanisms underlying the sleep regulation are unknown. Here, we used optogenetic tools to identify the key roles of VLPO astrocytes in sleep promotion. Optogenetic stimulation of VLPO astrocytes increased sleep duration in the active phase in naturally sleep-waking adult male rats (n = 6); it also increased the extracellular ATP concentration (n = 3) and c-Fos expression (n = 3-4) in neurons within the VLPO. In vivo microdialysis analyses revealed an increase in the activity of VLPO astrocytes and ATP levels during sleep states (n = 4). Moreover, metabolic inhibition of VLPO astrocytes reduced ATP levels (n = 4) and diminished sleep duration (n = 4). We further show that tissue-nonspecific alkaline phosphatase (TNAP), an ATP-degrading enzyme, plays a key role in mediating the somnogenic effects of ATP released from astrocytes (n = 5). An appropriate sample size for all experiments was based on statistical power calculations. Our results, taken together, indicate that astrocyte-derived ATP may be hydrolyzed into adenosine by TNAP, which may in turn act on VLPO neurons to promote sleep.SIGNIFICANCE STATEMENT Glia have recently been at the forefront of neuroscience research. Emerging evidence illustrates that astrocytes, the most abundant glial cell type, are the functional determinants for fates of neurons and other glial cells in the central nervous system. In this study, we newly identified the pivotal role of hypothalamic ventrolateral preoptic (VLPO) astrocytes in the sleep regulation, and provide novel insights into the mechanisms underlying the astrocyte-mediated sleep regulation.
Keywords: astrocyte; gliotransmitters; optogenetics; sleep; ventrolateral preoptic area.
Copyright © 2020 the authors.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
