Respiratory Trajectories in Type 2 and 3 Spinal Muscular Atrophy in the iSMAC Cohort Study
- PMID: 33067401
- PMCID: PMC7905794
- DOI: 10.1212/WNL.0000000000011051
Respiratory Trajectories in Type 2 and 3 Spinal Muscular Atrophy in the iSMAC Cohort Study
Abstract
Objective: To describe the respiratory trajectories and their correlation with motor function in an international pediatric cohort of patients with type 2 and nonambulant type 3 spinal muscular atrophy (SMA).
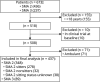
Methods: This was an 8-year retrospective observational study of patients in the International SMA Consortium (iSMAc) natural history study. We retrieved anthropometrics, forced vital capacity (FVC) absolute, FVC percent predicted (FVC%P), and noninvasive ventilation (NIV) requirement. Hammersmith Functional Motor Scale (HFMS) and revised Performance of Upper Limb (RULM) scores were correlated with respiratory function. We excluded patients in interventional clinical trials and on nusinersen commercial therapy.
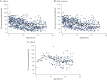
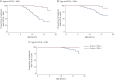
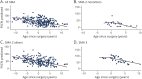
Results: There were 437 patients with SMA: 348 with type 2 and 89 with nonambulant type 3. Mean age at first visit was 6.9 (±4.4) and 11.1 (±4) years. In SMA type 2, FVC%P declined by 4.2%/y from 5 to 13 years, followed by a slower decline (1.0%/y). In type 3, FVC%P declined by 6.3%/y between 8 and 13 years, followed by a slower decline (0.9%/y). Thirty-nine percent with SMA type 2% and 9% with type 3 required NIV at a median age 5.0 (1.8-16.6) and 15.1 (13.8-16.3) years. Eighty-four percent with SMA type 2% and 80% with type 3 had scoliosis; 54% and 46% required surgery, which did not significantly affect respiratory decline. FVC%P positively correlated with HFMS and RULM scores in both subtypes.
Conclusions: In SMA type 2 and nonambulant type 3, lung function declines differently, with a common leveling after age 13 years. Lung and motor function correlated in both subtypes. Our data further define the milder SMA phenotypes and provide information to benchmark the long-term efficacy of new treatments for SMA.
Copyright © 2020 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
Similar articles
-
Therapeutic Role of Nusinersen on Respiratory Progression in Pediatric Patients With Spinal Muscular Atrophy Type 2 and Nonambulant Type 3.Neurol Clin Pract. 2024 Jun;14(3):e200298. doi: 10.1212/CPJ.0000000000200298. Epub 2024 May 17. Neurol Clin Pract. 2024. PMID: 38932995 Free PMC article.
-
Disease Trajectories in the Revised Hammersmith Scale in a Cohort of Untreated Patients with Spinal Muscular Atrophy types 2 and 3.J Neuromuscul Dis. 2024;11(3):665-677. doi: 10.3233/JND-230211. J Neuromuscul Dis. 2024. PMID: 38427497 Free PMC article.
-
Safety and Treatment Effects of Nusinersen in Longstanding Adult 5q-SMA Type 3 - A Prospective Observational Study.J Neuromuscul Dis. 2019;6(4):453-465. doi: 10.3233/JND-190416. J Neuromuscul Dis. 2019. PMID: 31594243 Free PMC article.
-
Drug treatment for spinal muscular atrophy types II and III.Cochrane Database Syst Rev. 2020 Jan 6;1(1):CD006282. doi: 10.1002/14651858.CD006282.pub5. Cochrane Database Syst Rev. 2020. PMID: 32006461 Free PMC article.
-
Functional and surgical treatments in patients with spinal muscular atrophy (SMA).Arch Pediatr. 2020 Dec;27(7S):7S35-7S39. doi: 10.1016/S0929-693X(20)30275-X. Arch Pediatr. 2020. PMID: 33357596 Review.
Cited by
-
Improved upper limb function in non-ambulant children with SMA type 2 and 3 during nusinersen treatment: a prospective 3-years SMArtCARE registry study.Orphanet J Rare Dis. 2022 Oct 23;17(1):384. doi: 10.1186/s13023-022-02547-8. Orphanet J Rare Dis. 2022. PMID: 36274155 Free PMC article.
-
What could be the function of the spinal muscular atrophy-causing protein SMN in macrophages?Front Immunol. 2024 May 28;15:1375428. doi: 10.3389/fimmu.2024.1375428. eCollection 2024. Front Immunol. 2024. PMID: 38863697 Free PMC article. Review.
-
Two-year efficacy and safety of risdiplam in patients with type 2 or non-ambulant type 3 spinal muscular atrophy (SMA).J Neurol. 2023 May;270(5):2531-2546. doi: 10.1007/s00415-023-11560-1. Epub 2023 Feb 3. J Neurol. 2023. PMID: 36735057 Free PMC article. Clinical Trial.
-
New therapies for spinal muscular atrophy: where we stand and what is next.Eur J Pediatr. 2023 Jul;182(7):2935-2942. doi: 10.1007/s00431-023-04883-8. Epub 2023 Apr 17. Eur J Pediatr. 2023. PMID: 37067602 Free PMC article. Review.
-
Therapeutic Role of Nusinersen on Respiratory Progression in Pediatric Patients With Spinal Muscular Atrophy Type 2 and Nonambulant Type 3.Neurol Clin Pract. 2024 Jun;14(3):e200298. doi: 10.1212/CPJ.0000000000200298. Epub 2024 May 17. Neurol Clin Pract. 2024. PMID: 38932995 Free PMC article.
References
-
- Lefebvre S, Bürglen L, Reboullet S, et al. . Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995;80:155–165. - PubMed
-
- Mercuri E, Bertini E, Iannaccone ST. Childhood spinal muscular atrophy: controversies and challenges. Lancet Neurol 2012;11:443–452. - PubMed
-
- Darras BT. Spinal muscular atrophies. Pediatr Clin North Am 2015;62:743–766. - PubMed
-
- Finkel RS, Mercuri E, Meyer OH, et al. . Diagnosis and management of spinal muscular atrophy, part 2: pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord 2018;28:197–207. - PubMed
