International Myeloma Working Group risk stratification model for smoldering multiple myeloma (SMM)
- PMID: 33067414
- PMCID: PMC7567803
- DOI: 10.1038/s41408-020-00366-3
International Myeloma Working Group risk stratification model for smoldering multiple myeloma (SMM)
Abstract
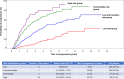
Smoldering multiple myeloma (SMM) is an asymptomatic precursor state of multiple myeloma (MM). Recently, MM was redefined to include biomarkers predicting a high risk of progression from SMM, thus necessitating a redefinition of SMM and its risk stratification. We assembled a large cohort of SMM patients meeting the revised IMWG criteria to develop a new risk stratification system. We included 1996 patients, and using stepwise selection and multivariable analysis, we identified three independent factors predicting progression risk at 2 years: serum M-protein >2 g/dL (HR: 2.1), involved to uninvolved free light-chain ratio >20 (HR: 2.7), and marrow plasma cell infiltration >20% (HR: 2.4). This translates into 3 categories with increasing 2-year progression risk: 6% for low risk (38%; no risk factors, HR: 1); 18% for intermediate risk (33%; 1 factor; HR: 3.0), and 44% for high risk (29%; 2-3 factors). Addition of cytogenetic abnormalities (t(4;14), t(14;16), +1q, and/or del13q) allowed separation into 4 groups (low risk with 0, low intermediate risk with 1, intermediate risk with 2, and high risk with ≥3 risk factors) with 6, 23, 46, and 63% risk of progression in 2 years, respectively. The 2/20/20 risk stratification model can be easily implemented to identify high-risk SMM for clinical research and routine practice and will be widely applicable.
Conflict of interest statement
M.-V.M. reports grants from Janssen, Celgene, Amgen, Takeda, Abbvie, GSK, Adaptive, EDO Mundipharma, Pharmamar, Roche, Seattle Genetics, outside the submitted work; S.K. reports research funding for clinical trials to the institution: Celgene, Takeda, Janssen, BMS, KITE, Merck, AbbVie, Medimmune, Novartis, Roche-Genentech, Amgen, Tenebio, Carsgen; and Consulting/Advisory Board participation: (with no personal payments) Celgene, Takeda, Janssen, AbbVie, Genentech, Amgen, Molecular Partners and (with personal payment) Oncopeptides, Gene Centrix, Cellectar. M.A.D. reports personal fees from Amgen, Celgene, Takeda, Janssen, and BMS, outside the submitted work. V.G.-C. reports grants from Janssen, personal fees from Advisory Board of Prothena, non-financial support from Janssen and Celgene, outside the submitted work; E.K. reports grants and personal fees from Amgen, personal fees and non-financial support from Genesis Pharma, grants, personal fees, and non-financial support from Janssen, personal fees from Takeda and Pfizer, during the conduct of the study; R.H. has nothing to disclose. C.F.d.L. reports grants, personal fees, and non-financial support from Janssen, Celgene, Takeda, and Amgen, outside the submitted work; G.J.M.: Advisory Boards from Celgene, BMS, Sanofi, Karyopharm, Janssen, Roche, and Genentech. G.M. has nothing to disclose. H.G. reports grants, personal fees and other fees from Celgene, Janssen, BMS, and Sanofi, grants and other from Chugai, grants from John Hopkins University and Dietmar Hopp Stiftung, other fees from Amgen, Molecular Partners, MSD, Mundipharma, and Art Tempi, personal fees and other fees from Takeda and Novartis, non-financial support and other fees from Adaptive Biotechnology, outside the submitted work. C.G. has nothing to disclose. A.G. reports grants and personal fees from Janssen, personal fees from Celgene, Amgen, and Takeda, outside the submitted work; C.K. has nothing to disclose. L.G. has nothing to disclose. M.H. has nothing to disclose. E.Z.: Advisory Board for Sanofi, BMS, Janssen, and Takeda. D.F. reports personal fees from Amgen, Janssen, Varifarma, Tecnofarma, Takeda, Sanofi, Glaxo, and Bristol Myers, outside the submitted work; X.L. has nothing to disclose. B.-S.K. reports grants from National Research Foundation of Korea (NRF), during the conduct of the study; G.E. has nothing to disclose. H.L. reports grants from Amgen, Takeda, personal fees from Amgen, Takeda, Janssen, BMS, Celgene, Seattle Genetics, and Sanofi, outside the submitted work; S.U. reports grants and personal fees from Amgen, Celgene, Sanofi, Seattle Genetics, Janssen, Takeda, SkylineDX, and Merck, personal fees from Abbvie and MundiPharma, grants from BMS and Pharmacyclics, outside the submitted work; C.-K.M. has nothing to disclose. M.Q.: employment from Janssen Research and Development; stock or other ownership from Janssen Research and Development. J.U.: employment from Janssen; stock or other ownership from Janssen. B.M.W. is an employee of Janssen Research & Development. S.V.R. has nothing to disclose. B.G.M.D.: Advisory Board for Amgen, Janssen, Celgene/BMS, and Takeda. J.S.-M. reports other fees from Amgen, BMS, Celgene, Janssen, MSD, Novartis, Takeda, Sanofi, Roche, GSK, AbbVie, and Karyopharm, outside the submitted work.
Figures



References
-
- Perez-Persona E, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood. 2007;110:2586–2592. doi: 10.1182/blood-2007-05-088443. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

