Gender differences in adverse event reports associated with antidiabetic drugs
- PMID: 33067519
- PMCID: PMC7567832
- DOI: 10.1038/s41598-020-74000-4
Gender differences in adverse event reports associated with antidiabetic drugs
Abstract
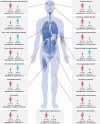
Little is known about gender-specific reporting of adverse events (AEs) associated with antidiabetic drugs. This study was to assess the gender-related difference in AEs reporting associated with antidiabetic agents. The number of antidiabetic drug-AE pairs associated was identified using the Korea Adverse Event Reporting System database. Prevalence of diabetes was estimated using the Health Insurance Review and Assessment Service-National Patients Sample database. Reporting rate per 10,000 people was calculated by dividing drug-AE pairs with the number of antidiabetic drug users by gender. Gender difference was presented with risk ratio (reporting rate ratio) of women to men. Antidiabetic agent-associated AEs were more frequently reported by women than men throughout body organs and drug classes. 13 out of 17 system organ class level disorders with significant gender differences were reported more often by women than men. By drug class, gender-specific reporting rates were observed in most of the drug classes, especially in newer classes such as glucagon-like peptide-1 analog (GLP1-RA), sodium glucose co-transporter-2 inhibitor (SGLT2i), and thiazolidinedione (TZD). Looking into preferred term level for each drug class, women dominated the reports of class-specific AEs of newer antidiabetic drugs such as urinary tract/genital infection (all reported by women) in SGLT2i, edema in TZD (risk ratio (RR) 12.56), and hyperglycemia in insulin users (RR 15.35). Gender differences in antidiabetic-associated AE reporting often attributed to women. Explanations for these different report levels by gender should be further investigated.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

