SPEG: a key regulator of cardiac calcium homeostasis
- PMID: 33067609
- PMCID: PMC8404462
- DOI: 10.1093/cvr/cvaa290
SPEG: a key regulator of cardiac calcium homeostasis
Abstract
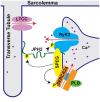
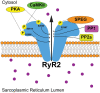
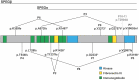
Proper cardiac Ca2+ homeostasis is essential for normal excitation-contraction coupling. Perturbations in cardiac Ca2+ handling through altered kinase activity has been implicated in altered cardiac contractility and arrhythmogenesis. Thus, a better understanding of cardiac Ca2+ handling regulation is vital for a better understanding of various human disease processes. 'Striated muscle preferentially expressed protein kinase' (SPEG) is a member of the myosin light chain kinase family that is key for normal cardiac function. Work within the last 5 years has revealed that SPEG has a crucial role in maintaining normal cardiac Ca2+ handling through maintenance of transverse tubule formation and phosphorylation of junctional membrane complex proteins. Additionally, SPEG has been causally impacted in human genetic diseases such as centronuclear myopathy and dilated cardiomyopathy as well as in common acquired cardiovascular disease such as heart failure and atrial fibrillation. Given the rapidly emerging role of SPEG as a key cardiac Ca2+ regulator, we here present this review in order to summarize recent findings regarding the mechanisms of SPEG regulation of cardiac excitation-contraction coupling in both physiology and human disease. A better understanding of the roles of SPEG will be important for a more complete comprehension of cardiac Ca2+ regulation in physiology and disease.
Keywords: Atrial fibrillation; Cardiomyopathy; Centronuclear myopathy; Excitation–contraction coupling; Heart failure; JPH2; Ryanodine receptor; SERCA2a; Striated muscle preferentially expressed protein kinase.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2020. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Heijman J, Dewenter M, El-Armouche A, Dobrev D.. Function and regulation of serine/threonine phosphatases in the healthy and diseased heart. J Mol Cell Cardiol 2013;64:90–98. - PubMed
-
- Vlahos CJ, McDowell SA, Clerk A.. Kinases as therapeutic targets for heart failure. Nat Rev Drug Discov 2003;2:99–113. - PubMed
-
- Goette A, Lendeckel U, Klein HU.. Signal transduction systems and atrial fibrillation. Cardiovasc Res 2002;54:247–258. - PubMed
-
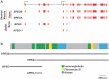
- Quick AP, Wang Q, Philippen LE, Barreto-Torres G, Chiang DY, Beavers D, Wang G, Khalid M, Reynolds JO, Campbell HM, Showell J, McCauley MD, Scholten A, Wehrens XH.. SPEG (striated muscle preferentially expressed protein kinase) is essential for cardiac function by regulating junctional membrane complex activity. Circ Res 2017;120:110–119. - PMC - PubMed
-
- Hsieh CM, Yoshizumi M, Endege WO, Kho CJ, Jain MK, Kashiki S, de los Santos R, Lee WS, Perrella MA, Lee ME.. APEG-1, a novel gene preferentially expressed in aortic smooth muscle cells, is down-regulated by vascular injury. J Biol Chem 1996;271:17354–17359. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

