The physiological and pathological functions of VEGFR3 in cardiac and lymphatic development and related diseases
- PMID: 33067626
- PMCID: PMC8262640
- DOI: 10.1093/cvr/cvaa291
The physiological and pathological functions of VEGFR3 in cardiac and lymphatic development and related diseases
Abstract
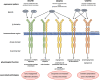
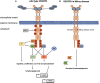
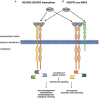
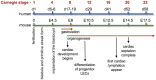
Vascular endothelial growth factor receptors (VEGFRs) are part of the evolutionarily conserved VEGF signalling pathways that regulate the development and maintenance of the body's cardiovascular and lymphovascular systems. VEGFR3, encoded by the FLT4 gene, has an indispensable and well-characterized function in development and establishment of the lymphatic system. Autosomal dominant VEGFR3 mutations, that prevent the receptor functioning as a homodimer, cause one of the major forms of hereditary primary lymphoedema; Milroy disease. Recently, we and others have shown that FLT4 variants, distinct to those observed in Milroy disease cases, predispose individuals to Tetralogy of Fallot, the most common cyanotic congenital heart disease, demonstrating a novel function for VEGFR3 in early cardiac development. Here, we examine the familiar and emerging roles of VEGFR3 in the development of both lymphovascular and cardiovascular systems, respectively, compare how distinct genetic variants in FLT4 lead to two disparate human conditions, and highlight the research still required to fully understand this multifaceted receptor.
Keywords: Angiogenesis and lymphangiogenesis; Congenital heart disease; Heart development; Primary lymphoedema; Vascular endothelial growth factor receptors.
© The Author(s) 2020. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

References
-
- Levick JR, Michel CC.. Microvascular fluid exchange and the revised starling principle. Cardiovasc Res 2010;87:198–210. - PubMed
-
- McAloon CJ, Boylan LM, Hamborg T, Stallard N, Osman F, Lim PB, Hayat SA.. The changing face of cardiovascular disease 2000-2012: an analysis of the world health organisation global health estimates data. Int J Cardiol 2016;224:256–264. - PubMed
-
- Green A. Outcomes of congenital heart disease: a review. Pediatr Nurs 2004;30:280–284. - PubMed
-
- Griffiths C, Barker J, Bleiker T, Chalmers R, Creamer D.. Rook's Textbook of Dermatology. Chichester, West Sussex; Hoboken, NJ: John Wiley & Sons Inc.; 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

