Incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy
- PMID: 33067632
- PMCID: PMC8016631
- DOI: 10.1182/blood.2020007878
Incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy
Abstract
The risk of venous thromboembolism (VTE) and arterial thromboembolism (ATE) associated with immune checkpoint inhibitors is currently unclear. Our aim was to quantify the risk of VTE/ATE in patients with cancer treated with immune checkpoint inhibitors, explore clinical impact, and investigate potential clinical risk factors. Patients treated with immune checkpoint inhibitors at the Medical University of Vienna from 2015 to 2018 were identified using in-house pharmacy records (n = 672; most frequent entities: 30.4% melanoma, 24.1% non-small cell lung cancer; 86% stage IV disease). A retrospective chart review was performed to screen for VTE and/or ATE. Cumulative incidences and between-group differences were estimated in competing-risk analysis. The impact of VTE/ATE on mortality was studied by multistate modelling. Over a median follow-up of 8.5 months, 47 VTEs and 9 ATEs were observed. Cumulative incidences of VTE and ATE were 12.9% (95% confidence interval [CI], 8.2-18.5) and 1.8% (95% CI, 0.7-3.6). Occurrence of VTE was associated with increased mortality (transition hazard ratio, 3.09; 95% CI, 2.07-4.60). History of VTE predicted VTE occurrence (subdistribution hazard ratio [SHR], 3.69; 95% CI, 2.00-6.81), and distant metastasis was nonsignificantly associated with VTE risk (SHR, 1.71; 95% CI, 0.62-4.73). No association of VTE with Eastern Cooperative Oncology Group performance status, Charlson comorbidity index, or Khorana score was observed, and rates of VTE were comparable between tumor types and checkpoint-inhibitory agents. In conclusion, patients with cancer under immune checkpoint inhibitor therapy are at high risk of thromboembolism, especially VTE. Furthermore, VTE occurrence was associated with increased mortality.
© 2021 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: C.H. reports speaker honoraria from Amgen, BMS, MSD, Novartis, and Roche and advisory board participation for Amgen, Astra Zeneca, BMS, Inzyte, MSD, Novartis, Pierre Fabre, and Roche. T.F. reports honoraria from MSD, Merck Darmstadt, Roche, BMS, Accord, Sanofi, and Boehringer Ingelheim and advisory board participation for MSD, Merck Darmstadt, Amgen, Pfizer, and Sanofi. S.Z.-M. received honoraria for advisory boards and/or lectures from Boehringer Ingelheim, Merck Sharp & Dohme, Bristol Myers Squibb, Roche, AstraZeneca, Takeda, and Pfizer and research support granted by Merck Sharp & Dohme. M.P. has received honoraria for lectures, consultation or advisory board participation from Bayer, Bristol Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, and Tocagen. I.P. reports honoraria for lectures and advisory board meetings from Bayer AG, Boehringer Ingelheim, Daiichi Sanchyo, and BMS/ Pfizer. C.A. reports honoraria for lectures from Bayer, Daiichi Sankyo, BMS/Pfizer, and Sanofi and participation in advisory boards for Bayer, Boehringer Ingelheim, Daiichi Sankyo, and BMS/Pfizer. The remaining authors declare no competing financial interests.
Figures

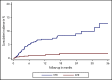
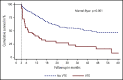
Comment in
-
Checkpoint inhibitors and thrombosis: what's up?Blood. 2021 Mar 25;137(12):1569-1570. doi: 10.1182/blood.2020009480. Blood. 2021. PMID: 33764429 No abstract available.
References
-
- Cohen AT, Katholing A, Rietbrock S, Bamber L, Martinez C. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. A population-based cohort study. Thromb Haemost. 2017;117(1):57-65. - PubMed
-
- Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: burden, mechanisms, and management. Thromb Haemost. 2017;117(2):219-230. - PubMed
-
- Seng S, Liu Z, Chiu SK, et al. . Risk of venous thromboembolism in patients with cancer treated with Cisplatin: a systematic review and meta-analysis. J Clin Oncol. 2012;30(35):4416-4426. - PubMed
-
- Proverbs-Singh T, Chiu SK, Liu Z, et al. . Arterial thromboembolism in cancer patients treated with cisplatin: a systematic review and meta-analysis. J Natl Cancer Inst. 2012;104(23):1837-1840. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

