Wip1 controls the translocation of the chromosomal passenger complex to the central spindle for faithful mitotic exit
- PMID: 33067654
- PMCID: PMC11072438
- DOI: 10.1007/s00018-020-03665-x
Wip1 controls the translocation of the chromosomal passenger complex to the central spindle for faithful mitotic exit
Abstract
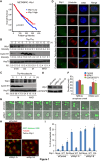
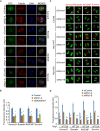
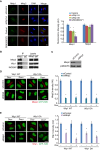
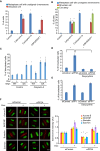
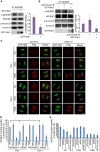
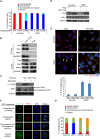
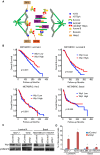
Dramatic cellular reorganization in mitosis critically depends on the timely and temporal phosphorylation of a broad range of proteins, which is mediated by the activation of the mitotic kinases and repression of counteracting phosphatases. The mitosis-to-interphase transition, which is termed mitotic exit, involves the removal of mitotic phosphorylation by protein phosphatases. Although protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) drive this reversal in animal cells, the phosphatase network associated with ordered bulk dephosphorylation in mitotic exit is not fully understood. Here, we describe a new mitotic phosphatase relay in which Wip1/PPM1D phosphatase activity is essential for chromosomal passenger complex (CPC) translocation to the anaphase central spindle after release from the chromosome via PP1-mediated dephosphorylation of histone H3T3. Depletion of endogenous Wip1 and overexpression of the phosphatase-dead mutant disturbed CPC translocation to the central spindle, leading to failure of cytokinesis. While Wip1 was degraded in early mitosis, its levels recovered in anaphase and the protein functioned as a Cdk1-counteracting phosphatase at the anaphase central spindle and midbody. Mechanistically, Wip1 dephosphorylated Thr-59 in inner centromere protein (INCENP), which, subsequently bound to MKLP2 and recruited other components to the central spindle. Furthermore, Wip1 overexpression is associated with the overall survival rate of patients with breast cancer, suggesting that Wip1 not only functions as a weak oncogene in the DNA damage network but also as a tumor suppressor in mitotic exit. Altogether, our findings reveal that sequential dephosphorylation of mitotic phosphatases provides spatiotemporal regulation of mitotic exit to prevent tumor initiation and progression.
Keywords: Aurora B; Checkpoint; DNA damage response; Homeostasis; MKLP1.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Switching of INCENP paralogs controls transitions in mitotic chromosomal passenger complex functions.Cell Cycle. 2019 Sep;18(17):2006-2025. doi: 10.1080/15384101.2019.1634954. Epub 2019 Jul 15. Cell Cycle. 2019. PMID: 31306061 Free PMC article.
-
Molecular basis of MKLP2-dependent Aurora B transport from chromatin to the anaphase central spindle.J Cell Biol. 2020 Jul 6;219(7):e201910059. doi: 10.1083/jcb.201910059. J Cell Biol. 2020. PMID: 32356865 Free PMC article.
-
Analysis of mitotic phosphorylation of borealin.BMC Cell Biol. 2007 Jan 22;8:5. doi: 10.1186/1471-2121-8-5. BMC Cell Biol. 2007. PMID: 17241471 Free PMC article.
-
Late mitotic functions of Aurora kinases.Chromosoma. 2017 Feb;126(1):93-103. doi: 10.1007/s00412-016-0594-5. Epub 2016 Apr 22. Chromosoma. 2017. PMID: 27106516 Review.
-
Getting out of mitosis: spatial and temporal control of mitotic exit and cytokinesis by PP1 and PP2A.FEBS Lett. 2019 Oct;593(20):2908-2924. doi: 10.1002/1873-3468.13595. Epub 2019 Sep 18. FEBS Lett. 2019. PMID: 31494926 Review.
Cited by
-
The Aurora kinase B relocation blocker LXY18 triggers mitotic catastrophe selectively in malignant cells.PLoS One. 2023 Oct 30;18(10):e0293283. doi: 10.1371/journal.pone.0293283. eCollection 2023. PLoS One. 2023. PMID: 37903144 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

