Effect of Ergothioneine on 7-Ketocholesterol-Induced Endothelial Injury
- PMID: 33067719
- PMCID: PMC7567423
- DOI: 10.1007/s12017-020-08620-4
Effect of Ergothioneine on 7-Ketocholesterol-Induced Endothelial Injury
Abstract
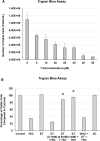
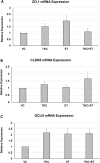
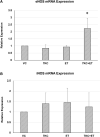
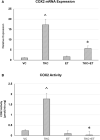
Ergothioneine (ET) is a naturally occurring antioxidant that is synthesized by non-yeast fungi and certain bacteria. ET is not synthesized by animals, including humans, but is avidly taken up from the diet, especially from mushrooms. In the current study, we elucidated the effect of ET on the hCMEC/D3 human brain endothelial cell line. Endothelial cells are exposed to high levels of the cholesterol oxidation product, 7-ketocholesterol (7KC), in patients with cardiovascular disease and diabetes, and this process is thought to mediate pathological inflammation. 7KC induces a dose-dependent loss of cell viability and an increase in apoptosis and necrosis in the endothelial cells. A relocalization of the tight junction proteins, zonula occludens-1 (ZO-1) and claudin-5, towards the nucleus of the cells was also observed. These effects were significantly attenuated by ET. In addition, 7KC induces marked increases in the mRNA expression of pro-inflammatory cytokines, IL-1β IL-6, IL-8, TNF-α and cyclooxygenase-2 (COX2), as well as COX2 enzymatic activity, and these were significantly reduced by ET. Moreover, the cytoprotective and anti-inflammatory effects of ET were significantly reduced by co-incubation with an inhibitor of the ET transporter, OCTN1 (VHCL). This shows that ET needs to enter the endothelial cells to have a protective effect and is unlikely to act via extracellular neutralizing of 7KC. The protective effect on inflammation in brain endothelial cells suggests that ET might be useful as a nutraceutical for the prevention or management of neurovascular diseases, such as stroke and vascular dementia. Moreover, the ability of ET to cross the blood-brain barrier could point to its usefulness in combatting 7KC that is produced in the CNS during neuroinflammation, e.g. after excitotoxicity, in chronic neurodegenerative diseases, and possibly COVID-19-related neurologic complications.
Keywords: 7-ketocholesterol; Anti-inflammatory; Antioxidant; Blood–brain barrier; COVID-19; COX2; Claudin-5; Coronavirus; Cyclooxygenase; Cytokines; Eicosanoids; Ergothioneine; Free radicals; Inflammation; Mushrooms; NF-kB; Oxidative stress; Oxysterols; TNF-α; Tight junction proteins; ZO-1.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures





References
-
- Anticoli S, Arciello M, Mancinetti A, De Martinis M, Ginaldi L, Iuliano L, et al. 7-ketocholesterol and 5,6-secosterol modulate differently the stress-activated mitogen-activated protein kinases (MAPKs) in liver cells. Journal of Cellular Physiology. 2010;222(3):586–595. doi: 10.1002/jcp.21972. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

