Developmental and reproductive safety evaluation of AV7909 anthrax vaccine candidate in rats
- PMID: 33067910
- PMCID: PMC7821328
- DOI: 10.1002/bdr2.1815
Developmental and reproductive safety evaluation of AV7909 anthrax vaccine candidate in rats
Abstract
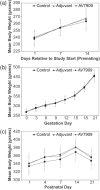
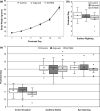
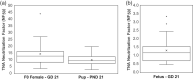
The AV7909 vaccine, consists of the Anthrax Vaccine Adsorbed (AVA) bulk drug substance and the immunostimulatory Toll-like receptor 9 agonist oligodeoxynucleotide adjuvant CPG 7909. The purpose of this research was to evaluate the potential maternal, reproductive, and developmental toxicity of AV7909 in rats to support licensure for use in women of childbearing potential. Groups of first generation (F0 ) female Sprague Dawley rats were dosed by intramuscular injection with water for injection, adjuvant or AV7909 at a volume of 0.5 ml/dose. Each rat received three vaccinations: 14 days prior to start of the mating period, on the first day of the mating period and on gestation day (GD) 7. There was no maternal mortality. Body weights, weight gain, and food consumption were comparable between groups. Findings in F0 females were limited to transient injection site edema and nodules consistent with immunostimulatory effects of the vaccine and adjuvant. Administration of AV7909 did not affect mating, fertility, pregnancy, embryo-fetal viability, growth, or morphologic development, parturition, maternal care of offspring or postnatal survival, growth, or development. There was no evidence of systemic inflammation in pregnant rats, based on evaluation of serum concentrations of the acute phase proteins alpha-2-macroglobulin and alpha-1-acid glycoprotein on GD 21. Anthrax lethal toxin-neutralizing antibodies were detected in AV7909-vaccinated F0 females. The antibodies were also detected in the sera of fetuses and F1 pups. Exposure of the fetuses and pups to maternally derived anthrax lethal toxin-neutralizing antibodies was not associated with developmental toxicity.
Keywords: AV7909; anthrax vaccine; developmental toxicity; rat; reproductive toxicity.
© 2020 The Authors. Birth Defects Research published by Wiley Periodicals LLC.
Conflict of interest statement
BB, JS, JR, BI, and VS are current or former employees of Emergent BioSolutions, Inc. EM, JB and AS are employees of Battelle, a Contract Research Organization where safety studies have been performed on a contractual basis for Emergent BioSolutions, Inc.
Figures


Similar articles
-
Evaluation of the AV7909 Anthrax Vaccine Toxicity in Sprague Dawley Rats Following Three Intramuscular Administrations.Int J Toxicol. 2021 Oct;40(5):442-452. doi: 10.1177/10915818211031239. Epub 2021 Jul 19. Int J Toxicol. 2021. PMID: 34281421 Free PMC article.
-
Repeat Dose Toxicity Study of the AV7909 Anthrax Vaccine Candidate in Juvenile Rats.Int J Toxicol. 2020 Jul 21:1091581820941412. doi: 10.1177/1091581820941412. Online ahead of print. Int J Toxicol. 2020. PMID: 32691648
-
Correlation between anthrax lethal toxin neutralizing antibody levels and survival in guinea pigs and nonhuman primates vaccinated with the AV7909 anthrax vaccine candidate.Vaccine. 2017 Sep 5;35(37):4952-4959. doi: 10.1016/j.vaccine.2017.07.076. Epub 2017 Jul 31. Vaccine. 2017. PMID: 28774566 Free PMC article.
-
Comparative immunogenicity and efficacy of thermostable (lyophilized) and liquid formulation of anthrax vaccine candidate AV7909.Vaccine. 2019 Oct 8;37(43):6356-6361. doi: 10.1016/j.vaccine.2019.09.015. Epub 2019 Sep 14. Vaccine. 2019. PMID: 31530467 Free PMC article.
-
Randomized, double-blind, placebo-controlled, safety and immunogenicity study of 4 formulations of Anthrax Vaccine Adsorbed plus CPG 7909 (AV7909) in healthy adult volunteers.Vaccine. 2013 Jun 26;31(30):3051-8. doi: 10.1016/j.vaccine.2013.04.063. Epub 2013 May 10. Vaccine. 2013. PMID: 23701746 Free PMC article. Clinical Trial.
Cited by
-
Evaluation of the AV7909 Anthrax Vaccine Toxicity in Sprague Dawley Rats Following Three Intramuscular Administrations.Int J Toxicol. 2021 Oct;40(5):442-452. doi: 10.1177/10915818211031239. Epub 2021 Jul 19. Int J Toxicol. 2021. PMID: 34281421 Free PMC article.
References
-
- Astroff, A. B. , Ray, S. E. , Rowe, L. M. , Hilbish, K. G. , Linville, A. L. , Stutz, J. P. , & Breslin, W. J. (2002). Frozen‐sectioning yields similar results as traditional methods for fetal cephalic examination in the rat. Teratology, 66(2), 77–84. - PubMed
-
- Barrow, M. V. , & Taylor, W. J. (1969). A rapid method for detecting malformations in rat fetuses. Journal of Morphology, 127(3), 291–305. - PubMed
-
- CDC . (2010). Use of anthrax vaccine in the United States: Recommendations of the advisory committee on immunization practices (ACIP), 2009. MMWR, 59, 1–30. - PubMed
-
- Conlin, A. M. S. , Sevick, C. J. , Gumbs, G. R. , Khodr, Z. G. , & Bukowinski, A. T. (2017). Safety of inadvertent anthrax vaccination during pregnancy: An analysis of birth defects in the U.S. military population, 2003‐2010. Vaccine, 35(34), 4414–4420. - PubMed
-
- Conover, W. J. (1980). Practical nonparametric statistics In Bradley R. A., Hunter J. S., Kendall D. G., & Watson G. S. (Eds.), Wiley series in probability and mathematical statistics (2nd ed.). New York, Chichester, Brisbane, Toronto: John Wiley & Sons.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

