Safety and Efficacy of Omaveloxolone in Friedreich Ataxia (MOXIe Study)
- PMID: 33068037
- PMCID: PMC7894504
- DOI: 10.1002/ana.25934
Safety and Efficacy of Omaveloxolone in Friedreich Ataxia (MOXIe Study)
Erratum in
-
Correction to Safety and Efficacy of Omaveloxolone in Friedreich Ataxia (MOXIe Study).Ann Neurol. 2023 Dec;94(6):1190. doi: 10.1002/ana.26808. Epub 2023 Oct 5. Ann Neurol. 2023. PMID: 37795909 Free PMC article. No abstract available.
Abstract
Objective: Friedreich ataxia (FA) is a progressive genetic neurodegenerative disorder with no approved treatment. Omaveloxolone, an Nrf2 activator, improves mitochondrial function, restores redox balance, and reduces inflammation in models of FA. We investigated the safety and efficacy of omaveloxolone in patients with FA.
Methods: We conducted an international, double-blind, randomized, placebo-controlled, parallel-group, registrational phase 2 trial at 11 institutions in the United States, Europe, and Australia (NCT02255435, EudraCT2015-002762-23). Eligible patients, 16 to 40 years of age with genetically confirmed FA and baseline modified Friedreich's Ataxia Rating Scale (mFARS) scores between 20 and 80, were randomized 1:1 to placebo or 150mg per day of omaveloxolone. The primary outcome was change from baseline in the mFARS score in those treated with omaveloxolone compared with those on placebo at 48 weeks.
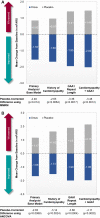
Results: One hundred fifty-five patients were screened, and 103 were randomly assigned to receive omaveloxolone (n = 51) or placebo (n = 52), with 40 omaveloxolone patients and 42 placebo patients analyzed in the full analysis set. Changes from baseline in mFARS scores in omaveloxolone (-1.55 ± 0.69) and placebo (0.85 ± 0.64) patients showed a difference between treatment groups of -2.40 ± 0.96 (p = 0.014). Transient reversible increases in aminotransferase levels were observed with omaveloxolone without increases in total bilirubin or other signs of liver injury. Headache, nausea, and fatigue were also more common among patients receiving omaveloxolone.
Interpretation: In the MOXIe trial, omaveloxolone significantly improved neurological function compared to placebo and was generally safe and well tolerated. It represents a potential therapeutic agent in FA. ANN NEUROL 2021;89:212-225.
© 2020 The Authors. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
This work was sponsored and funded by Reata Pharmaceuticals, which is developing omaveloxolone for clinical applications. M.P.C., C.J.M., M.O., and A.G. are employees of Reata Pharmaceuticals.
Figures



References
-
- Lynch DR, Farmer JM, Balcer LJ, Wilson RB. Friedreich ataxia: effects of genetic understanding on clinical evaluation and therapy. Arch Neurol 2002;59:743–747. - PubMed
-
- Pandolfo M. Friedreich ataxia. Arch Neurol 2008;65:1296–1303. - PubMed
-
- Galea CA, Huq A, Lockhart PJ, et al. Compound heterozygous FXN mutations and clinical outcome in Friedreich ataxia. Ann Neurol 2016;79:485–495. - PubMed
-
- Delatycki MB, Bidichandani SI. Friedreich ataxia—pathogenesis and implications for therapies. Neurobiol Dis 2019;132:104606. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

