Oncogenic KRAS-expressing organoids with biliary epithelial stem cell properties give rise to biliary tract cancer in mice
- PMID: 33068050
- PMCID: PMC8088913
- DOI: 10.1111/cas.14703
Oncogenic KRAS-expressing organoids with biliary epithelial stem cell properties give rise to biliary tract cancer in mice
Abstract
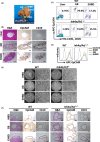
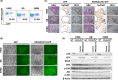
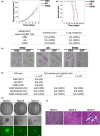
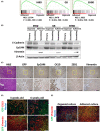
Biliary tract cancer (BTC) arises from biliary epithelial cells (BECs) and includes intrahepatic cholangiocarcinoma (IHCC), gallbladder cancer (GC), and extrahepatic cholangiocarcinoma (EHCC). Although frequent KRAS mutations and epigenetic changes at the INK4A/ARF locus have been identified, the molecular pathogenesis of BTC is unclear and the development of corresponding anticancer agents remains inadequate. We isolated epithelial cell adhesion molecule (EpCAM)-positive BECs from the mouse intrahepatic bile duct, gallbladder, and extrahepatic bile duct, and established organoids derived from these cells. Introduction of activated KRAS and homozygous deletion of Ink4a/Arf in the cells of each organoid type conferred the ability to form lethal metastatic adenocarcinoma with differentiated components and a pronounced desmoplastic reaction on cell transplantation into syngeneic mice, indicating that the manipulated cells correspond to BTC-initiating cells. The syngeneic mouse models recapitulate the pathological features of human IHCC, GC, and EHCC, and they should therefore prove useful for the investigation of BTC carcinogenesis and the development of new therapeutic strategies. Tumor cells isolated from primary tumors formed organoids in three-dimensional culture, and serial syngeneic transplantation of these cells revealed that their cancer stem cell properties were supported by organoid culture, but not by adherent culture. Adherent culture thus attenuated tumorigenic activity as well as the expression of both epithelial and stem cell markers, whereas the expression of epithelial-mesenchymal transition (EMT)-related transcription factor genes and mesenchymal cell markers was induced. Our data show that organoid culture is important for maintenance of epithelial cell characteristics, stemness, and tumorigenic activity of BTC-initiating cells.
Keywords: biliary tract cancer; cancer stem cell; cholangiocarcinoma; epithelial-mesenchymal transition; organoid culture.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69‐90. - PubMed
-
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273‐1281. - PubMed
-
- Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003‐1010. - PubMed
MeSH terms
Substances
Grants and funding
- KAKENHI 20K08968/Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan
- KAKENHI 22130007/Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan
- Translational Research Network Program, Research on Applying Health Technology, and Research on Rare and Intractable Diseases grants from the Japan Agency for Medical Research and Development
- Keio Gijuku Academic Development Funds
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

