Adipocyte-specific deletion of Tcf7l2 induces dysregulated lipid metabolism and impairs glucose tolerance in mice
- PMID: 33068125
- PMCID: PMC7567653
- DOI: 10.1007/s00125-020-05292-4
Adipocyte-specific deletion of Tcf7l2 induces dysregulated lipid metabolism and impairs glucose tolerance in mice
Abstract
Aims/hypothesis: Transcription factor 7-like 2 (TCF7L2) is a downstream effector of the Wnt/β-catenin signalling pathway implicated in type 2 diabetes risk through genome-wide association studies. Although its expression is critical for adipocyte development, the potential roles of changes in adipose tissue TCF7L2 levels in diabetes risk are poorly defined. Here, we investigated whether forced changes in Tcf7l2 expression in adipocytes affect whole body glucose or lipid metabolism and crosstalk between disease-relevant tissues.
Methods: Tcf7l2 was selectively ablated in mature adipocytes in C57BL/6J mice using Cre recombinase under Adipoq promoter control to recombine Tcf7l2 alleles floxed at exon 1 (referred to as aTCF7L2 mice). aTCF7L2 mice were fed normal chow or a high-fat diet for 12 weeks. Glucose and insulin sensitivity, as well as beta cell function, were assessed in vivo and in vitro. Levels of circulating NEFA, selected hormones and adipokines were measured using standard assays.
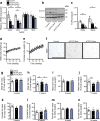
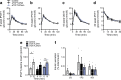
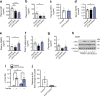
Results: Reduced TCF7L2 expression in adipocytes altered glucose tolerance and insulin secretion in male but not in female mice. Thus, on a normal chow diet, male heterozygote knockout mice (aTCF7L2het) exhibited impaired glucose tolerance at 16 weeks (p = 0.03) and increased fat mass (1.4 ± 0.1-fold, p = 0.007) but no changes in insulin secretion. In contrast, male homozygote knockout (aTCF7L2hom) mice displayed normal body weight but impaired oral glucose tolerance at 16 weeks (p = 0.0001). These changes were mechanistically associated with impaired in vitro glucose-stimulated insulin secretion (decreased 0.5 ± 0.1-fold vs control mice, p = 0.02) and decreased levels of the incretins glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide (0.6 ± 0.1-fold and 0.4 ± 0.1-fold vs control mice, p = 0.04 and p < 0.0001, respectively). Circulating levels of plasma NEFA and fatty acid binding protein 4 were increased by 1.3 ± 0.1-fold and 1.8 ± 0.3-fold vs control mice (p = 0.03 and p = 0.05, respectively). Following exposure to a high-fat diet for 12 weeks, male aTCF7L2hom mice exhibited reduced in vivo glucose-stimulated insulin secretion (0.5 ± 0.1-fold vs control mice, p = 0.02).
Conclusions/interpretation: Loss of Tcf7l2 gene expression selectively in adipocytes leads to a sexually dimorphic phenotype, with impairments not only in adipocytes, but also in pancreatic islet and enteroendocrine cells in male mice only. Our findings suggest novel roles for adipokines and incretins in the effects of diabetes-associated variants in TCF7L2, and further illuminate the roles of TCF7L2 in glucose homeostasis and diabetes risk. Graphical abstract.
Keywords: Adipocyte; Beta cell; Fatty acid; Incretin; Insulin; Mouse; TCF7L2; Type 2 diabetes.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

