Targeting miR-124/Ferroportin signaling ameliorated neuronal cell death through inhibiting apoptosis and ferroptosis in aged intracerebral hemorrhage murine model
- PMID: 33068460
- PMCID: PMC7681046
- DOI: 10.1111/acel.13235
Targeting miR-124/Ferroportin signaling ameliorated neuronal cell death through inhibiting apoptosis and ferroptosis in aged intracerebral hemorrhage murine model
Erratum in
-
Correction to "Targeting miR-124/ Ferroportin Signaling Ameliorated Neuronal Cell Death Through Inhibiting Apoptosis and Ferroptosis in Aged Intracerebral Hemorrhage Murine Model".Aging Cell. 2025 Mar;24(3):e70006. doi: 10.1111/acel.70006. Epub 2025 Feb 13. Aging Cell. 2025. PMID: 39945592 Free PMC article. No abstract available.
Abstract
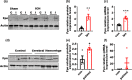
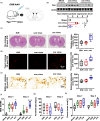
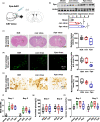
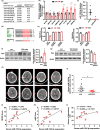
Incidence of intracerebral hemorrhage (ICH) and brain iron accumulation increases with age. Excess iron accumulation in brain tissues post-ICH induces oxidative stress and neuronal damage. However, the mechanisms underlying iron deregulation in ICH, especially in the aged ICH model have not been well elucidated. Ferroportin1 (Fpn) is the only identified nonheme iron exporter in mammals to date. In our study, we reported that Fpn was significantly upregulated in perihematomal brain tissues of both aged ICH patients and mouse model. Fpn deficiency induced by injecting an adeno-associated virus (AAV) overexpressing cre recombinase into aged Fpn-floxed mice significantly worsened the symptoms post-ICH, including hematoma volume, cell apoptosis, iron accumulation, and neurologic dysfunction. Meanwhile, aged mice pretreated with a virus overexpressing Fpn showed significant improvement of these symptoms. Additionally, based on prediction of website tools, expression level of potential miRNAs in ICH tissues and results of luciferase reporter assays, miR-124 was identified to regulate Fpn expression post-ICH. Higher serum miR-124 levels were correlated with poor neurologic scores of aged ICH patients. Administration of miR-124 antagomir enhanced Fpn expression and attenuated iron accumulation in aged mice model. Both apoptosis and ferroptosis, but not necroptosis, were regulated by miR-124/Fpn signaling manipulation. Our study demonstrated the critical role of miR-124/Fpn signaling in iron metabolism and neuronal death post-ICH in aged murine model. Thus, Fpn upregulation or miR-124 inhibition might be promising therapeutic approachs for this disease.
Keywords: Fpn; apoptosis; ferroptosis; intracerebral hemorrhage; iron; miR-124.
© 2020 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

