Construction and validation of a database of head models for functional imaging of the neonatal brain
- PMID: 33068482
- PMCID: PMC7814762
- DOI: 10.1002/hbm.25242
Construction and validation of a database of head models for functional imaging of the neonatal brain
Abstract
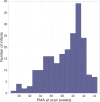
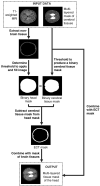
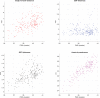
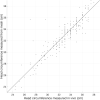
The neonatal brain undergoes dramatic structural and functional changes over the last trimester of gestation. The accuracy of source localisation of brain activity recorded from the scalp therefore relies on accurate age-specific head models. Although an age-appropriate population-level atlas could be used, detail is lost in the construction of such atlases, in particular with regard to the smoothing of the cortical surface, and so such a model is not representative of anatomy at an individual level. In this work, we describe the construction of a database of individual structural priors of the neonatal head using 215 individual-level datasets at ages 29-44 weeks postmenstrual age from the Developing Human Connectome Project. We have validated a method to segment the extra-cerebral tissue against manual segmentation. We have also conducted a leave-one-out analysis to quantify the expected spatial error incurred with regard to localising functional activation when using a best-matching individual from the database in place of a subject-specific model; the median error was calculated to be 8.3 mm (median absolute deviation 3.8 mm). The database can be applied for any functional neuroimaging modality which requires structural data whereby the physical parameters associated with that modality vary with tissue type and is freely available at www.ucl.ac.uk/dot-hub.
Keywords: atlas; database; neonatal; structural prior.
© 2020 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Alexander, B. , Murray, A. L. , Loh, W. Y. , Matthews, L. G. , Adamson, C. , Beare, R. , … Thompson, D. K. (2017). A new neonatal cortical and subcortical brain atlas: The Melbourne Children's Regional Infant Brain (M‐CRIB) atlas. NeuroImage, 147, 841–851. 10.1016/j.neuroimage.2016.09.068 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

