Biological effects of inhaled hydraulic fracturing sand dust. IV. Pulmonary effects
- PMID: 33068619
- PMCID: PMC7736927
- DOI: 10.1016/j.taap.2020.115284
Biological effects of inhaled hydraulic fracturing sand dust. IV. Pulmonary effects
Abstract
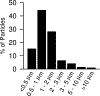
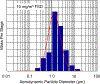
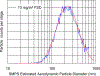
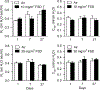
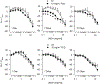
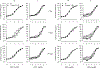
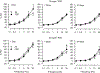
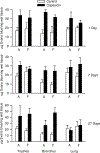
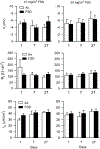
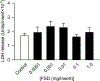
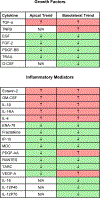
Hydraulic fracturing creates fissures in subterranean rock to increase the flow and retrieval of natural gas. Sand ("proppant") in fracking fluid injected into the well bore maintains fissure patency. Fracking sand dust (FSD) is generated during manipulation of sand to prepare the fracking fluid. Containing respirable crystalline silica, FSD could pose hazards similar to those found in work sites where silica inhalation induces lung disease such as silicosis. This study was performed to evaluate the possible toxic effects following inhalation of a FSD (FSD 8) in the lung and airways. Rats were exposed (6 h/d × 4 d) to 10 or 30 mg/m3 of a FSD collected at a gas well, and measurements were performed 1, 7, 27 and, in one series of experiments, 90 d post-exposure. The following ventilatory and non-ventilatory parameters were measured in vivo and/or in vitro: 1) lung mechanics (respiratory system resistance and elastance, tissue damping, tissue elastance, Newtonian resistance and hysteresivity); 2) airway reactivity to inhaled methacholine (MCh); airway epithelium integrity (isolated, perfused trachea); airway efferent motor nerve activity (electric field stimulation in vitro); airway smooth muscle contractility; ion transport in intact and cultured epithelium; airway effector and sensory nerves; tracheal particle deposition; and neurogenic inflammation/vascular permeability. FSD 8 was without large effect on most parameters, and was not pro-inflammatory, as judged histologically and in cultured epithelial cells, but increased reactivity to inhaled MCh at some post-exposure time points and affected Na+ transport in airway epithelial cells.
Keywords: Fracking sand dust; Hydraulic fracturing; Lung; Pulmonary toxicity.
Published by Elsevier Inc.
Conflict of interest statement
Conflict of Interest Statement
The authors declare that they have no conflicts of interest in relation to this publication.
Figures








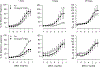


References
-
- Anderson SE, Shane H, Long C, Marrocco A, Lukomska E, Roberts JR, Marshall N, and Fedan JS, 2020. Biological effects of inhaled hydraulic fracturing sand dust. VIII. Immunotoxicity. Toxicol Appl Pharmacol (Manuscript submitted to this journal as a tandem paper to accompany this manuscript.) - PMC - PubMed
-
- Castranova V, Porter D, Millecchia L, Ma JY, Hubbs AF, Teass A, 2002. Effect of inhaled crystalline silica in a rat model: time course of pulmonary reactions. Mol Cell Biochem 234–235, 177–184. - PubMed
-
- Dai J, Gilks B, Price K, Churg A, 1998. Mineral dusts directly induce epithelial and interstitial fibrogenic mediators and matrix components in the airway wall. Am J Respir Crit Care Med 158, 1907–1913. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

