Recovery of reward function in problematic substance users using a combination of robotics, electrophysiology, and TMS
- PMID: 33068631
- PMCID: PMC10353476
- DOI: 10.1016/j.ijpsycho.2020.08.008
Recovery of reward function in problematic substance users using a combination of robotics, electrophysiology, and TMS
Abstract
Background: Theoretical and empirical work suggest that addictive drugs potentiate dopaminergic reinforcement learning signals and disrupt the reward function of its neural targets, including the anterior midcingulate cortex (aMCC) and the basal ganglia. Here, we aim to use prefrontal 10-Hz TMS to enhance aMCC reward activity and reward learning by the basal ganglia in problematic substance users.
Methods: 22 problematic substance users were randomized into an Active and SHAM (coil flipped) TMS group. We recorded the reward positivity-an electrophysiological signal believed to index sensitivity of the aMCC to rewards-while participants engaged in 4 blocks (100 trials per block) of a reward-based choice task. A robotic arm positioned a TMS coil over a prefrontal cortex target, and 50 pulses were delivered at 10-Hz before every 10 trials of blocks 2-4 (1500 pulses, 400 trials). Participants then completed a decision-making task that is diagnostic of striatal dopamine dysfunction.
Results: The present study revealed three main findings. First, both groups failed to elicit a reward positivity during the first two task blocks. Second, applying robot-assisted TMS enhanced the amplitude of the reward positivity in the Active group, but not the SHAM group, across the last two task blocks. Third, the Active group performed relatively better at reward-based learning than the SHAM group.
Conclusion: These results demonstrate that 10-Hz TMS is successful in modulating the reward function of the aMCC and basal ganglia in problematic substance users, which may have utility in the treatment of reward-related neural dysfunction commonly associated with substance use disorders.
Keywords: Anterior midcingulate cortex; Cognitive control; Decision-making; Reward positivity; Substance use disorder; TMS.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest
The authors report no conflict of interest.
Figures

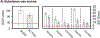
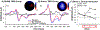

References
-
- Baker TE, Holroyd CB, 2009. Which way do I go? Neural activation in response to feedback and spatial processing in a virtual T-maze. Cereb. Cortex 19, 1708–1722. - PubMed
-
- Baker TE, Holroyd CB, 2011. Dissociated roles of the anterior cingulate cortex in reward and conflict processing as revealed by the feedback error-related negativity and N200. Biol. Psychol. 87, 25–34. - PubMed
-
- Baker TE, Stockwell T, Barnes G, Holroyd CB, 2011. Individual differences in substance dependence: at the intersection of brain, behaviour and cognition. Addict. Biol. 16, 458–466. - PubMed
-
- Baker TE, Stockwell T, Holroyd CB, 2013. Constraints on decision making: implications from genetics, personality, and addiction. Cogn. Affect. Behav. Neurosci. 13, 417–436. - PubMed
-
- Baker TE, Stockwell T, Barnes G, Haesevoets R, Holroyd CB, 2016a. Reward sensitivity of ACC as an intermediate phenotype between DRD4–521T and substance misuse. J. Cogn. Neurosci. 28, 460–471. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

