Sexual dimorphism in insulin resistance in a metabolic syndrome rat model
- PMID: 33069157
- PMCID: PMC7583132
- DOI: 10.1530/EC-20-0288
Sexual dimorphism in insulin resistance in a metabolic syndrome rat model
Abstract
Objective: We assessed the sex-specific differences in the molecular mechanisms of insulin resistance in muscle and adipose tissue, in a MS rat model induced by a high sucrose diet.
Methods: Male, female, and ovariectomized female Wistar rats were randomly distributed in control and high-sucrose diet (HSD) groups, supplemented for 24 weeks with 20% sucrose in the drinking water. At the end, we assessed parameters related to MS, analyzing the effects of the HSD on critical nodes of the insulin signaling pathway in muscle and adipose tissue.
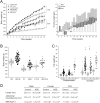
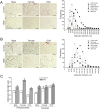
Results: At the end of the treatment, HSD groups of both sexes developed obesity, with a 15, 33 and 23% of body weight gain in male, female, and OVX groups respectively, compared with controls; mainly related to hypertrophy of peripancreatic and gonadal adipose tissue. They also developed hypertriglyceridemia, and liver steatosis, with the last being worse in the HSD females. Compared to the control groups, HSD rats had higher IL1B and TNFA levels and insulin resistance. HSD females were more intolerant to glucose than HSD males. Our observations suggest that insulin resistance mechanisms include an increase in phosphorylated AKT(S473) form in HSD male and female groups and a decrease in phosphorylated P70S6K1(T389) in the HSD male groups from peripancreatic adipose tissue. While in gonadal adipose tissue the phosphorylated form of AKT decreased in HSD females, but not in HSD males. Finally, HSD groups showed a reduction in p-AKT levels in gastrocnemius muscle.
Conclusion: A high-sucrose diet induces MS and insulin resistance with sex-associated differences and in a tissue-specific manner.
Keywords: insulin resistance; metabolic syndrome; obesity; sexual dimorphism.
Figures






References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

