Potential research ethics violations against an indigenous tribe in Ecuador: a mixed methods approach
- PMID: 33069227
- PMCID: PMC7568418
- DOI: 10.1186/s12910-020-00542-x
Potential research ethics violations against an indigenous tribe in Ecuador: a mixed methods approach
Abstract
Background: Biomedical and ethnographic studies among indigenous people are common practice in health and geographical research. Prior health research misconduct has been documented, particularly when obtaining genetic material. The objective of this study was to crossmatch previously published data with the perceptions of the Waorani peoples about the trading of their genetic material and other biological samples.
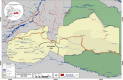
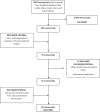
Methods: We conducted a mixed methods study design using a tailored 15-item questionnaire in 72 participants and in-depth interviews in 55 participants belonging to 20 Waorani communities about their experiences and perceptions of participating in biomedical research projects. Additionally, we conducted a systematic review of the literature in order to crossmatch the published results of studies stating the approval of an ethics committee and individual consent within their work.
Results: A total of 40 men (60%) and 32 women (40%), with a mean age of 57 ± 15 years agreed to be interviewed for inclusion. Five main categories around the violation of good clinical practices were identified, concerning the obtention of blood samples from a recently contacted Waorani native community within the Amazonian region of Ecuador. These themes are related to the lack of adequate communication between community members and researchers as well as the voluntariness to participate in health research. Additionally, over 40 years, a total of 38 manuscripts related to the use of biological samples in Waorani indigenous people were published. The majority of the studies (68%) did not state within their article obtaining research ethics board approval, and 71% did not report obtaining the informed consent of the participants prior to the execution of the project.
Conclusion: Clinical Research on the Waorani community in the Ecuadorian Amazon basin has been performed on several occasions. Unfortunately, the majority of these projects did not follow the appropriate ethical and professional standards in either reporting the results or fulfilling them. The results of our investigation suggest that biological material, including genetic material, has been used by researchers globally, with some omitting the minimum information required to guarantee transparency and good clinical practices. We highlight the importance of stating ethics within research to avoid breaches in research transparency.
Keywords: Consent; Ecuador; Indigenous populations; Research ethics board; Waorani.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Baeta M, Núñez C, González-Andrade F, Sosa C, Casalod Y, Bolea M, et al. Mitochondrial analysis revealed high homogeneity in the Waorani population—The last nomadic group of hunter-gatherers from Ecuador. Forensic Sci Int Genet Suppl Ser. 2009;2:313–314. doi: 10.1016/j.fsigss.2009.08.025. - DOI
-
- Pringle H. Uncontacted tribe in Brazil emerges from isolation. American Association for the Advancement of Science; 2014. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

