Paired Nasopharyngeal and Deep Lung Testing for Severe Acute Respiratory Syndrome Coronavirus-2 Reveals a Viral Gradient in Critically Ill Patients: A Multicenter Study
- PMID: 33069724
- PMCID: PMC7557286
- DOI: 10.1016/j.chest.2020.10.017
Paired Nasopharyngeal and Deep Lung Testing for Severe Acute Respiratory Syndrome Coronavirus-2 Reveals a Viral Gradient in Critically Ill Patients: A Multicenter Study
Figures

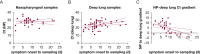
Comment in
-
Finding an Evidence-Based and Clinically Important Role for BAL in the Setting of Suspected SARS-Cov-2 Infection.Chest. 2021 Apr;159(4):1680-1681. doi: 10.1016/j.chest.2020.11.037. Epub 2021 Apr 6. Chest. 2021. PMID: 34022001 Free PMC article. No abstract available.
-
Response.Chest. 2021 Apr;159(4):1681-1682. doi: 10.1016/j.chest.2020.11.038. Epub 2021 Apr 6. Chest. 2021. PMID: 34022002 Free PMC article. No abstract available.
References
-
- Intensive Care National Audit and Research Network COVID-19 report. https://www.icnarc.org/DataServices/Attachments/Download/7bc41c30-efc2-e...
-
- Wölfel R., Corman V.M., Guggemos W., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

