The discovery of novel antitrypanosomal 4-phenyl-6-(pyridin-3-yl)pyrimidines
- PMID: 33070078
- PMCID: PMC7762786
- DOI: 10.1016/j.ejmech.2020.112871
The discovery of novel antitrypanosomal 4-phenyl-6-(pyridin-3-yl)pyrimidines
Abstract
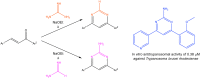
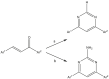
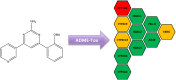
Human African trypanosomiasis, or sleeping sickness, is a neglected tropical disease caused by Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense which seriously affects human health in Africa. Current therapies present limitations in their application, parasite resistance, or require further clinical investigation for wider use. Our work herein describes the design and syntheses of novel antitrypanosomal 4-phenyl-6-(pyridin-3-yl)pyrimidines, with compound 13, the 4-(2-methoxyphenyl)-6-(pyridine-3-yl)pyrimidin-2-amine demonstrating an IC50 value of 0.38 μM and a promising off-target ADME-Tox profile in vitro. In silico molecular target investigations showed rhodesain to be a putative candidate, supported by STD and WaterLOGSY NMR experiments, however, in vitro evaluation of compound 13 against rhodesain exhibited low experimental inhibition. Therefore, our reported library of drug-like pyrimidines present promising scaffolds for further antikinetoplastid drug development for both phenotypic and target-based drug discovery.
Keywords: ADME-Tox; Antitrypanosomal; Docking; Human african trypanosomiasis; Pyrimidines; Rhodesain; Sleeping sickness; Trypanosoma brucei rhodesiense.
Crown Copyright © 2020. Published by Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Baker C.H., Welburn S.C. The long wait for a new drug for human African trypanosomiasis. Trends Parasitol. 2018;34(10):818–827. - PubMed
-
- Fairlamb A.H., Horn D. Melarsoprol resistance in African trypanosomiasis. Trends Parasitol. 2018;34(6):481–492. - PubMed
-
- Mesu V.K.B.K., Kalonji W.M., Bardonneau C., Mordt O.V., Blesson S., Simon F., Delhomme S., Bernhard S., Kuziena W., Lubaki J.-P.F., Vuvu S.L., Ngima P.N., Mbembo H.M., Ilunga M., Bonama A.K., Heradi J.A., Solomo J.L.L., Mandula G., Badibabi L.K., Dama F.R., Lukula P.K., Tete D.N., Lumbala C., Scherrer B., Strub-Wourgaft N., Tarral A. Oral fexinidazole for late-stage African Trypanosoma brucei gambiense trypanosomiasis: a pivotal multicentre, randomised, non-inferiority trial. Lancet. 2018;391(10116):144–154. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

