Neurogenic tachykinin mechanisms in experimental nephritis of rats
- PMID: 33070237
- PMCID: PMC7691313
- DOI: 10.1007/s00424-020-02469-z
Neurogenic tachykinin mechanisms in experimental nephritis of rats
Abstract
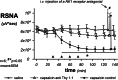
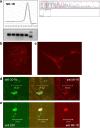
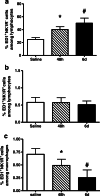
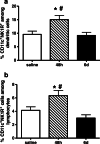
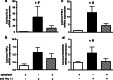
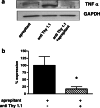
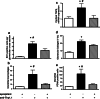
We demonstrated earlier that renal afferent pathways combine very likely "classical" neural signal transduction to the central nervous system and a substance P (SP)-dependent mechanism to control sympathetic activity. SP content of afferent sensory neurons is known to mediate neurogenic inflammation upon release. We tested the hypothesis that alterations in SP-dependent mechanisms of renal innervation contribute to experimental nephritis. Nephritis was induced by OX-7 antibodies in rats, 6 days later instrumented for recording of blood pressure (BP), heart rate (HR), drug administration, and intrarenal administration (IRA) of the TRPV1 agonist capsaicin to stimulate afferent renal nerve pathways containing SP and electrodes for renal sympathetic nerve activity (RSNA). The presence of the SP receptor NK-1 on renal immune cells was assessed by FACS. IRA capsaicin decreased RSNA from 62.4 ± 5.1 to 21.6 ± 1.5 mV s (*p < 0.05) in controls, a response impaired in nephritis. Suppressed RSNA transiently but completely recovered after systemic administration of a neurokinin 1 (NK1-R) blocker. NK-1 receptors occurred mainly on CD11+ dendritic cells (DCs). An enhanced frequency of CD11c+NK1R+ cell, NK-1 receptor+ macrophages, and DCs was assessed in nephritis. Administration of the NK-1R antagonist aprepitant during nephritis reduced CD11c+NK1R+ cells, macrophage infiltration, renal expression of chemokines, and markers of sclerosis. Hence, SP promoted renal inflammation by weakening sympathoinhibitory mechanisms, while at the same time, substance SP released intrarenally from afferent nerve fibers aggravated immunological processes i.e. by the recruitment of DCs.
Keywords: Dendritic cells; Nephritis; Renal innervation; Sympathetic nerve activity; Tachykinins.
Figures

References
-
- Bernard C (1859) Leçons sur les Propriétés et les Altérations Pathologiques des Liquides de L’Organisme. Bailliére et Fils, Paris: vol 2, 170
-
- Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

