Efficacy and safety of tafamidis doses in the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT) and long-term extension study
- PMID: 33070419
- PMCID: PMC8048553
- DOI: 10.1002/ejhf.2027
Efficacy and safety of tafamidis doses in the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT) and long-term extension study
Abstract
Aims: Tafamidis is an effective treatment for transthyretin amyloid cardiomyopathy (ATTR-CM) in the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT). While ATTR-ACT was not designed for a dose-specific assessment, further analysis from ATTR-ACT and its long-term extension study (LTE) can guide determination of the optimal dose.
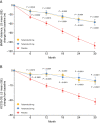
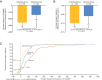
Methods and results: In ATTR-ACT, patients were randomized (2:1:2) to tafamidis 80 mg, 20 mg, or placebo for 30 months. Patients completing ATTR-ACT could enrol in the LTE (with placebo-treated patients randomized to tafamidis 80 or 20 mg; 2:1) and all patients were subsequently switched to high-dose tafamidis. All-cause mortality was assessed in ATTR-ACT combined with the LTE (median follow-up 51 months). In ATTR-ACT, the combination of all-cause mortality and cardiovascular-related hospitalizations over 30 months was significantly reduced with tafamidis 80 mg (P = 0.0030) and 20 mg (P = 0.0048) vs. placebo. All-cause mortality vs. placebo was reduced with tafamidis 80 mg [Cox hazards model (95% confidence interval): 0.690 (0.487-0.979), P = 0.0378] and 20 mg [0.715 (0.450-1.137), P = 0.1564]. The mean (standard error) change in N-terminal pro-B-type natriuretic peptide from baseline to Month 30 was -1170.51 (587.31) (P = 0.0468) with tafamidis 80 vs. 20 mg. In ATTR-ACT combined with the LTE there was a significantly greater survival benefit with tafamidis 80 vs. 20 mg [0.700 (0.501-0.979), P = 0.0374]. Incidence of adverse events in both tafamidis doses were comparable to placebo.
Conclusion: Tafamidis, both 80 and 20 mg, effectively reduced mortality and cardiovascular-related hospitalizations in patients with ATTR-CM. The longer-term survival data and the lack of dose-related safety concerns support tafamidis 80 mg as the optimal dose.
Clinical trial registration: ClinicalTrials.gov NCT01994889; NCT02791230.
Keywords: Biomarkers; Clinical trial; Mortality; Transthyretin amyloid cardiomyopathy.
© 2020 The Authors. European Journal of Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Figures

Comment in
-
Letter regarding the article 'Efficacy and safety of tafamidis doses in the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT) and long-term extension study'.Eur J Heart Fail. 2021 Apr;23(4):681-682. doi: 10.1002/ejhf.2049. Epub 2020 Nov 12. Eur J Heart Fail. 2021. PMID: 33150648 No abstract available.
-
Reply to the letter regarding the article 'Efficacy and safety of tafamidis doses in the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT) and long-term extension study'.Eur J Heart Fail. 2021 Jun;23(6):1057-1058. doi: 10.1002/ejhf.2074. Epub 2021 Feb 16. Eur J Heart Fail. 2021. PMID: 33340199 Free PMC article. No abstract available.
-
Tafamidis is entering the clinical arena for the treatment of transthyretin-related cardiomyopathy: certainties and unmet needs.Eur J Heart Fail. 2021 Feb;23(2):286-289. doi: 10.1002/ejhf.2104. Epub 2021 Jan 26. Eur J Heart Fail. 2021. PMID: 33443315 No abstract available.
-
New effective treatment options reinforce disease awareness: the case of transthyretin cardiac amyloidosis.Eur J Heart Fail. 2021 Feb;23(2):290-292. doi: 10.1002/ejhf.2111. Epub 2021 Feb 11. Eur J Heart Fail. 2021. PMID: 33527646 No abstract available.
References
-
- Rapezzi C, Quarta CC, Riva L, Longhi S, Gallelli I, Lorenzini M, Ciliberti P, Biagini E, Salvi F, Branzi A. Transthyretin‐related amyloidoses and the heart: a clinical overview. Nat Rev Cardiol 2010;7:398–408. - PubMed
-
- Almeida MR, Hesse A, Steinmetz A, Maisch B, Altland K, Linke RP, Gawinowicz MA, Saraiva MJ. Transthyretin Leu 68 in a form of cardiac amyloidosis. Basic Res Cardiol 1991;86:567–571. - PubMed
-
- Jacobson DR, Pastore RD, Yaghoubian R, Kane I, Gallo G, Buck FS, Buxbaum JN. Variant‐sequence transthyretin (isoleucine 122) in late‐onset cardiac amyloidosis in black Americans. N Engl J Med 1997;336:466–473. - PubMed
-
- Ranløv I, Alves IL, Ranløv PJ, Husby G, Costa PP, Saraiva MJ. A Danish kindred with familial amyloid cardiomyopathy revisited: identification of a mutant transthyretin‐methionine111 variant in serum from patients and carriers. Am J Med 1992;93:3–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

