KEAP1/NFE2L2 Mutations Predict Lung Cancer Radiation Resistance That Can Be Targeted by Glutaminase Inhibition
- PMID: 33071215
- PMCID: PMC7710558
- DOI: 10.1158/2159-8290.CD-20-0282
KEAP1/NFE2L2 Mutations Predict Lung Cancer Radiation Resistance That Can Be Targeted by Glutaminase Inhibition
Abstract
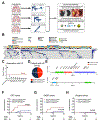
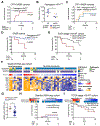
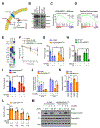
Tumor genotyping is not routinely performed in localized non-small cell lung cancer (NSCLC) due to lack of associations of mutations with outcome. Here, we analyze 232 consecutive patients with localized NSCLC and demonstrate that KEAP1 and NFE2L2 mutations are predictive of high rates of local recurrence (LR) after radiotherapy but not surgery. Half of LRs occurred in tumors with KEAP1/NFE2L2 mutations, indicating that they are major molecular drivers of clinical radioresistance. Next, we functionally evaluate KEAP1/NFE2L2 mutations in our radiotherapy cohort and demonstrate that only pathogenic mutations are associated with radioresistance. Furthermore, expression of NFE2L2 target genes does not predict LR, underscoring the utility of tumor genotyping. Finally, we show that glutaminase inhibition preferentially radiosensitizes KEAP1-mutant cells via depletion of glutathione and increased radiation-induced DNA damage. Our findings suggest that genotyping for KEAP1/NFE2L2 mutations could facilitate treatment personalization and provide a potential strategy for overcoming radioresistance conferred by these mutations. SIGNIFICANCE: This study shows that mutations in KEAP1 and NFE2L2 predict for LR after radiotherapy but not surgery in patients with NSCLC. Approximately half of all LRs are associated with these mutations and glutaminase inhibition may allow personalized radiosensitization of KEAP1/NFE2L2-mutant tumors.This article is highlighted in the In This Issue feature, p. 1775.
©2020 American Association for Cancer Research.
Figures

References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. - PubMed
-
- Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–99. - PMC - PubMed
-
- Senthi S, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol. 2012;13:802–9. - PubMed
-
- Ball D, Mai GT, Vinod S, Babington S, Ruben J, Kron T, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019;20:494–503. - PubMed

