Multicomponent Reactions: "Kinderleicht"
- PMID: 33071311
- PMCID: PMC7558297
- DOI: 10.1021/acs.jchemed.0c00290
Multicomponent Reactions: "Kinderleicht"
Abstract
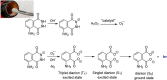
A demonstration experiment of the synthesis of a novel tetrazole derivative via a multicomponent reaction (Ugi tetrazole four component reaction, UT-4CR) bearing a luminol moiety and a subsequent exploitation of its chemiluminescent properties is described. A complex product is generated in just one simple step, so simple that children can do it: "kinderleicht", German for dead easy. Students are stimulated, inspired, and involved in a multilevel active learning process using the Steps to Inquiry framework as a metacognitive tool that raises student awareness regarding scientific process and prompts them to ask their own questions discussing the merits of a mechanism and evaluating its effectiveness before they start their own cycles of inquiry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Bybee R.; Taylor J.; Gardner A.; Scotter P.; Carlson J.; Westbrook A.; Landes N. The BSCS 5E Instructional Model: Origins, Effectiveness, and Applications. BSCS 2006, 5, 88–98.
-
- Brown A. L.; Campione J. C.. Guided Discovery in a Community of Learners. Classroom Lessons: Integrating Cognitive Theory and Classroom Practice; The MIT Press: Cambridge, MA, 1994; pp 229–270.
-
- Krathwohl D. R. A. Revision of Bloom’s Taxonomy: An Overview. Theory Pract. 2002, 41 (4), 212–218. 10.1207/s15430421tip4104_2. - DOI
-
- Shea J. H. National Science Education Standards. J. Geol. Educ. 1995, 43 (2), 102–102. 10.5408/0022-1368-43.2.102. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
