Fabrication of Injectable Micro-Scale Opto-Electronically Transduced Electrodes (MOTEs) for Physiological Monitoring
- PMID: 33071528
- PMCID: PMC7560984
- DOI: 10.1109/jmems.2020.2999496
Fabrication of Injectable Micro-Scale Opto-Electronically Transduced Electrodes (MOTEs) for Physiological Monitoring
Abstract
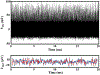
In vivo, chronic neural recording is critical to understand the nervous system, while a tetherless, miniaturized recording unit can render such recording minimally invasive. We present a tetherless, injectable micro-scale opto-electronically transduced electrode (MOTE) that is ~60μm × 30μm × 330μm, the smallest neural recording unit to date. The MOTE consists of an AlGaAs micro-scale light emitting diode (μLED) heterogeneously integrated on top of conventional 180nm complementary metal-oxide-semiconductor (CMOS) circuit. The MOTE combines the merits of optics (AlGaAs μLED for power and data uplink), and of electronics (CMOS for signal amplification and encoding). The optical powering and communication enable the extreme scaling while the electrical circuits provide a high temporal resolution (<100μs). This paper elaborates on the heterogeneous integration in MOTEs, a topic that has been touted without much demonstration on feasibility or scalability. Based on photolithography, we demonstrate how to build heterogenous systems that are scalable as well as biologically stable - the MOTEs can function in saline water for more than six months, and in a mouse brain for two months (and counting). We also present handling/insertion techniques for users (i.e. biologists) to deploy MOTEs with little or no extra training.
Keywords: CMOS post processing; electrophysiology; heterogenous integration; physiological monitoring; tetherless neural recording.
Figures
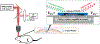
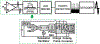





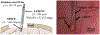

Similar articles
-
A 250 μm × 57 μm Microscale Opto-electronically Transduced Electrodes (MOTEs) for Neural Recording.IEEE Trans Biomed Circuits Syst. 2018 Dec;12(6):1256-1266. doi: 10.1109/TBCAS.2018.2876069. Epub 2018 Oct 15. IEEE Trans Biomed Circuits Syst. 2018. PMID: 30334768 Free PMC article.
-
A method for efficient, rapid, and minimally invasive implantation of individual non-functional motes with penetrating subcellular-diameter carbon fiber electrodes into rat cortex.bioRxiv [Preprint]. 2025 Feb 8:2025.02.05.636655. doi: 10.1101/2025.02.05.636655. bioRxiv. 2025. PMID: 39974888 Free PMC article. Preprint.
-
A Scalable and Low Stress Post-CMOS Processing Technique for Implantable Microsensors.Micromachines (Basel). 2020 Oct 5;11(10):925. doi: 10.3390/mi11100925. Micromachines (Basel). 2020. PMID: 33028005 Free PMC article.
-
Optimizing Nanoelectrode Arrays for Scalable Intracellular Electrophysiology.Acc Chem Res. 2018 Mar 20;51(3):600-608. doi: 10.1021/acs.accounts.7b00519. Epub 2018 Feb 13. Acc Chem Res. 2018. PMID: 29437381 Review.
-
New approaches for CMOS-based devices for large-scale neural recording.Curr Opin Neurobiol. 2015 Jun;32:31-7. doi: 10.1016/j.conb.2014.10.007. Epub 2014 Oct 30. Curr Opin Neurobiol. 2015. PMID: 25463562 Review.
Cited by
-
Fabrication and Assembly Techniques for Sub-mm Battery-Free Epicortical Implants.Micromachines (Basel). 2023 Feb 18;14(2):476. doi: 10.3390/mi14020476. Micromachines (Basel). 2023. PMID: 36838175 Free PMC article.
-
Integrated Micro-Devices for a Lab-in-Organoid Technology Platform: Current Status and Future Perspectives.Front Neurosci. 2022 Apr 26;16:842265. doi: 10.3389/fnins.2022.842265. eCollection 2022. Front Neurosci. 2022. PMID: 35557601 Free PMC article.
-
Tracking the Migration of Injectable Microdevices in the Rodent Brain Using a 9.4T Magnetic Resonance Imaging Scanner.Front Neurosci. 2021 Oct 5;15:738589. doi: 10.3389/fnins.2021.738589. eCollection 2021. Front Neurosci. 2021. PMID: 34675768 Free PMC article.
-
A conceptual advance that gives microrobots legs.Nature. 2020 Aug;584(7822):530-531. doi: 10.1038/d41586-020-02421-2. Nature. 2020. PMID: 32848217 No abstract available.
-
Highly stable integration of graphene Hall sensors on a microfluidic platform for magnetic sensing in whole blood.Microsyst Nanoeng. 2023 May 31;9:71. doi: 10.1038/s41378-023-00530-2. eCollection 2023. Microsyst Nanoeng. 2023. PMID: 37275264 Free PMC article.
References
-
- Bai Q, Wise K and Anderson D, “A high-yield microassembly structure for three-dimensional microelectrode arrays,” in IEEE Transactions on Biomedical Engineering, vol. 47, no. 3, pp. 281–289, March 2000. - PubMed
-
- Michon F, Aarts A, Holzhammer T, Ruther P, Borghs G, McNaughton B and Kloosterman F, “Integration of silicon-based neural probes and micro-drive arrays for chronic recording of large populations of neurons in behaving animals,” Journal of Neural Engineering, vol. 13, no. 4, p. 046018, June 2016. - PubMed
-
- Szarowski D, Andersen M, Retterer S, Spence A, Isaacson M, Craighead H, Turner J and Shain W, “Brain responses to micro-machined silicon devices,” Brain Research, vol. 983, no. 1-2, pp. 23–35, 2003. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
