Gene Therapy for Neurodegenerative Diseases: Slowing Down the Ticking Clock
- PMID: 33071748
- PMCID: PMC7530328
- DOI: 10.3389/fnins.2020.580179
Gene Therapy for Neurodegenerative Diseases: Slowing Down the Ticking Clock
Abstract
Gene therapy is an emerging and powerful therapeutic tool to deliver functional genetic material to cells in order to correct a defective gene. During the past decades, several studies have demonstrated the potential of AAV-based gene therapies for the treatment of neurodegenerative diseases. While some clinical studies have failed to demonstrate therapeutic efficacy, the use of AAV as a delivery tool has demonstrated to be safe. Here, we discuss the past, current and future perspectives of gene therapies for neurodegenerative diseases. We also discuss the current advances on the newly emerging RNAi-based gene therapies which has been widely studied in preclinical model and recently also made it to the clinic.
Keywords: AAV (adeno-associated virus); ALS; RNA interference (RNAi); gene therapy; neurodegenarative disease.
Copyright © 2020 Martier and Konstantinova.
Figures
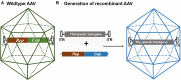

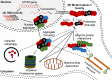
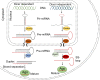

References
-
- Alnylam Pharmaceuticals (2020). Alnylam Announces Approval of GIVLAARI§(givosiran) in the European Union for the Treatment of Acute Hepatic Porphyria (AHP) in Adults and Adolescents. Avaliable at: https://investors.alnylam.com/press-release?id=24651 (accessed March 3, 2020).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

