Regulation of Angiotensin- Converting Enzyme 2 in Obesity: Implications for COVID-19
- PMID: 33071815
- PMCID: PMC7531362
- DOI: 10.3389/fphys.2020.555039
Regulation of Angiotensin- Converting Enzyme 2 in Obesity: Implications for COVID-19
Abstract
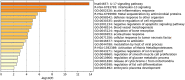
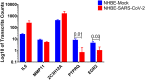
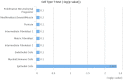
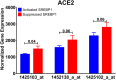
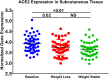
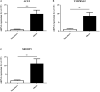
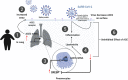
The ongoing COVID-19 pandemic is caused by the novel coronavirus SARS-CoV-2. Age, smoking, obesity, and chronic diseases such as cardiovascular disease and diabetes have been described as risk factors for severe complications and mortality in COVID-19. Obesity and diabetes are usually associated with dysregulated lipid synthesis and clearance, which can initiate or aggravate pulmonary inflammation and injury. It has been shown that for viral entry into the host cell, SARS-CoV-2 utilizes the angiotensin-converting enzyme 2 (ACE2) receptors present on the cells. We aimed to characterize how SARS-CoV-2 dysregulates lipid metabolism pathways in the host and the effect of dysregulated lipogenesis on the regulation of ACE2, specifically in obesity. In our study, through the re-analysis of publicly available transcriptomic data, we first found that lung epithelial cells infected with SARS-CoV-2 showed upregulation of genes associated with lipid metabolism, including the SOC3 gene, which is involved in the regulation of inflammation and inhibition of leptin signaling. This is of interest as viruses may hijack host lipid metabolism to allow the completion of their viral replication cycles. Furthermore, a dataset using a mouse model of diet-induced obesity showed a significant increase in Ace2 expression in the lungs, which negatively correlated with the expression of genes that code for sterol response element-binding proteins 1 and 2 (SREBP). Suppression of Srebp1 showed a significant increase in Ace2 expression in the lung. Moreover, ACE2 expression in human subcutaneous adipose tissue can be regulated through changes in diet. Validation of the in silico data revealed a higher expression of ACE2, TMPRSS2 and SREBP1 in vitro in lung epithelial cells from obese subjects compared to non-obese subjects. To our knowledge this is the first study to show upregulation of ACE2 and TMPRSS2 in obesity. In silico and in vitro results suggest that the dysregulated lipogenesis and the subsequently high ACE2 expression in obese patients might be the mechanism underlying the increased risk for severe complications in those patients when infected by SARS-CoV-2.
Keywords: ACE2; COVID-19; SARS-CoV-2; lipid metabolism; obesity.
Copyright © 2020 Al Heialy, Hachim, Senok, Gaudet, Abou Tayoun, Hamoudi, Alsheikh-Ali and Hamid.
Figures



References
-
- Blanco-Melo D., Nilsson-Payant B. E., Liu W.-C., Møller R., Panis M., Sachs D., et al. (2020). SARS-CoV-2 launches a unique transcriptional signature from in vitro, ex vivo, and in vivo systems. bioRxiv [Preprint]. 10.1101/2020.03.24.004655 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

