SP_0916 Is an Arginine Decarboxylase That Catalyzes the Synthesis of Agmatine, Which Is Critical for Capsule Biosynthesis in Streptococcus pneumoniae
- PMID: 33072045
- PMCID: PMC7531197
- DOI: 10.3389/fmicb.2020.578533
SP_0916 Is an Arginine Decarboxylase That Catalyzes the Synthesis of Agmatine, Which Is Critical for Capsule Biosynthesis in Streptococcus pneumoniae
Abstract
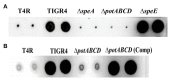
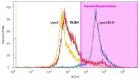
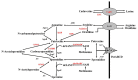
The global burden of invasive pneumococcal diseases, including pneumonia and sepsis, caused by Streptococcus pneumoniae, a Gram-positive bacterial pathogen, remains a major global health risk. The success of pneumococcus as a pathogen can be attributed to its ability to regulate the synthesis of capsular polysaccharide (CPS) during invasive disease. We previously reported that deletion of a putative lysine decarboxylase (LDC; ΔSP_0916) in pneumococcal serotype 4 (TIGR4) results in reduced CPS. SP_0916 locus is annotated as either an arginine or a LDC in pneumococcal genomes. In this study, by biochemical characterization of the recombinant SP_0916, we determined the substrate specificity of SP_0916 and show that it is an arginine decarboxylase (speA/ADC). We also show that deletion of the polyamine transporter (potABCD) predicted to import putrescine and spermidine results in reduced CPS, while deletion of spermidine synthase (speE) for the conversion of putrescine to spermidine had no impact on the capsule. Targeted metabolomics identified a correlation between reduced levels of agmatine and loss of capsule in ΔspeA and ΔpotABCD, while agmatine levels were comparable between the encapsulated TIGR4 and ΔspeE. Exogenous supplementation of agmatine restored CPS in both ΔpotABCD and ΔspeA. These results demonstrate that agmatine is critical for regulating the CPS, a predominant virulence factor in pneumococci.
Keywords: Streptococcus pneumoniae; agmatine; capsular polysaccharide; metabolomics; polyamines.
Copyright © 2020 Ayoola, Nakamya, Shack, Park, Lim, Lee, Ross, Eoh and Nanduri.
Figures


Similar articles
-
Arginine Decarboxylase Is Essential for Pneumococcal Stress Responses.Pathogens. 2021 Mar 2;10(3):286. doi: 10.3390/pathogens10030286. Pathogens. 2021. PMID: 33801541 Free PMC article.
-
Characterization of an Arginine Decarboxylase from Streptococcus pneumoniae by Ultrahigh-Performance Liquid Chromatography-Tandem Mass Spectrometry.Biomolecules. 2024 Apr 10;14(4):463. doi: 10.3390/biom14040463. Biomolecules. 2024. PMID: 38672479 Free PMC article.
-
Polyamine biosynthesis and transport mechanisms are crucial for fitness and pathogenesis of Streptococcus pneumoniae.Microbiology (Reading). 2011 Feb;157(Pt 2):504-515. doi: 10.1099/mic.0.042564-0. Epub 2010 Oct 21. Microbiology (Reading). 2011. PMID: 20966092
-
The expansive effects of polyamines on the metabolism and virulence of Streptococcus pneumoniae.Pneumonia (Nathan). 2021 Mar 25;13(1):4. doi: 10.1186/s41479-021-00082-x. Pneumonia (Nathan). 2021. PMID: 33762024 Free PMC article. Review.
-
Current trends in capsular polysaccharide biosynthesis of Streptococcus pneumoniae.Res Microbiol. 2000 Jul-Aug;151(6):429-35. doi: 10.1016/s0923-2508(00)00173-x. Res Microbiol. 2000. PMID: 10961455 Review.
Cited by
-
Arginine Decarboxylase Is Essential for Pneumococcal Stress Responses.Pathogens. 2021 Mar 2;10(3):286. doi: 10.3390/pathogens10030286. Pathogens. 2021. PMID: 33801541 Free PMC article.
-
The anti-inflammatory effects of cinnamyl alcohol on sepsis-induced mice via the NLRP3 inflammasome pathway.Ann Transl Med. 2022 Jan;10(2):48. doi: 10.21037/atm-21-6130. Ann Transl Med. 2022. PMID: 35282107 Free PMC article.
-
Characterization of an Arginine Decarboxylase from Streptococcus pneumoniae by Ultrahigh-Performance Liquid Chromatography-Tandem Mass Spectrometry.Biomolecules. 2024 Apr 10;14(4):463. doi: 10.3390/biom14040463. Biomolecules. 2024. PMID: 38672479 Free PMC article.
-
Impact of Difluoromethylornithine and AMXT 1501 on Gene Expression and Capsule Regulation in Streptococcus pneumoniae.Biomolecules. 2024 Feb 2;14(2):178. doi: 10.3390/biom14020178. Biomolecules. 2024. PMID: 38397415 Free PMC article.
-
Difluoromethylornithine (DFMO) and AMXT 1501 inhibit capsule biosynthesis in pneumococci.Sci Rep. 2022 Jul 12;12(1):11804. doi: 10.1038/s41598-022-16007-7. Sci Rep. 2022. PMID: 35821246 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

