A Novel, Orally Delivered Antibody Therapy and Its Potential to Prevent Clostridioides difficile Infection in Pre-clinical Models
- PMID: 33072047
- PMCID: PMC7537341
- DOI: 10.3389/fmicb.2020.578903
A Novel, Orally Delivered Antibody Therapy and Its Potential to Prevent Clostridioides difficile Infection in Pre-clinical Models
Abstract
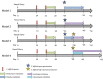
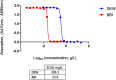
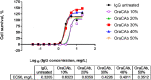
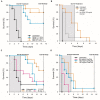
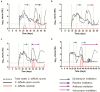
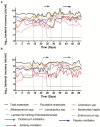
Clostridioides difficile infection (CDI) is a toxin-mediated infection in the gut and a major burden on healthcare facilities worldwide. We rationalized that it would be beneficial to design an antibody therapy that is delivered to, and is active at the site of toxin production, rather than neutralizing the circulating and luminal toxins after significant damage of the layers of the intestines has occurred. Here we describe a highly potent therapeutic, OraCAb, with high antibody titers and a formulation that protects the antibodies from digestion/inactivation in the gastrointestinal tract. The potential of OraCAb to prevent CDI in an in vivo hamster model and an in vitro human colon model was assessed. In the hamster model we optimized the ratio of the antibodies against each of the toxins produced by C. difficile (Toxins A and B). The concentration of immunoglobulins that is effective in a hamster model of CDI was determined. A highly significant difference in animal survival for those given an optimized OraCAb formulation versus an untreated control group was observed. This is the first study testing the effect of oral antibodies for treatment of CDI in an in vitro gut model seeded with a human fecal inoculum. Treatment with OraCAb successfully neutralized toxin production and did not interfere with the colonic microbiota in this model. Also, treatment with a combination of vancomycin and OraCAb prevented simulated CDI recurrence, unlike vancomycin therapy alone. These data demonstrate the efficacy of OraCAb formulation for the treatment of CDI in pre-clinical models.
Keywords: Clostridioides difficile infection (CDI); formulation protecting antibodies from digestion/inactivation; immunotherapy of CDI; in vitro human gut model of CDI; in vivo hamster model of CDI; oral antibodies.
Copyright © 2020 Roberts, Harris, Smith, Giles, Polak, Buckley, Clark, Ewin, Moura, Spitall, Shone, Wilcox, Chilton and Donev.
Figures

Similar articles
-
The role of the gut microbiome in colonization resistance and recurrent Clostridioides difficile infection.Therap Adv Gastroenterol. 2022 Nov 18;15:17562848221134396. doi: 10.1177/17562848221134396. eCollection 2022. Therap Adv Gastroenterol. 2022. PMID: 36425405 Free PMC article. Review.
-
Novel, non-colonizing, single-strain live biotherapeutic product ADS024 protects against Clostridioides difficile infection challenge in vivo.World J Gastrointest Pathophysiol. 2023 Aug 24;14(4):71-85. doi: 10.4291/wjgp.v14.i4.71. World J Gastrointest Pathophysiol. 2023. PMID: 37727283 Free PMC article.
-
Ridinilazole: a novel, narrow-spectrum antimicrobial agent targeting Clostridium (Clostridioides) difficile.Lett Appl Microbiol. 2022 Sep;75(3):526-536. doi: 10.1111/lam.13664. Epub 2022 Feb 11. Lett Appl Microbiol. 2022. PMID: 35119124 Free PMC article. Review.
-
Efficacy and Safety of RBX2660 in PUNCH CD3, a Phase III, Randomized, Double-Blind, Placebo-Controlled Trial with a Bayesian Primary Analysis for the Prevention of Recurrent Clostridioides difficile Infection.Drugs. 2022 Oct;82(15):1527-1538. doi: 10.1007/s40265-022-01797-x. Epub 2022 Oct 26. Drugs. 2022. PMID: 36287379 Free PMC article. Clinical Trial.
-
CDBN-YGXZ, a Novel Small-Molecule Drug, Shows Efficacy against Clostridioides difficile Infection and Recurrence in Mouse and Hamster Infection Models.Antimicrob Agents Chemother. 2023 May 17;67(5):e0170422. doi: 10.1128/aac.01704-22. Epub 2023 Apr 13. Antimicrob Agents Chemother. 2023. PMID: 37052498 Free PMC article.
Cited by
-
Immunization Strategies Against Clostridioides difficile.Adv Exp Med Biol. 2024;1435:117-150. doi: 10.1007/978-3-031-42108-2_7. Adv Exp Med Biol. 2024. PMID: 38175474 Review.
-
Application of recombinant antibodies for treatment of Clostridioides difficile infection: Current status and future perspective.Front Immunol. 2022 Aug 23;13:972930. doi: 10.3389/fimmu.2022.972930. eCollection 2022. Front Immunol. 2022. PMID: 36081500 Free PMC article. Review.
-
MiGut: A scalable in vitro platform for simulating the human gut microbiome-Development, validation and simulation of antibiotic-induced dysbiosis.Microb Biotechnol. 2023 Jun;16(6):1312-1324. doi: 10.1111/1751-7915.14259. Epub 2023 Apr 10. Microb Biotechnol. 2023. PMID: 37035991 Free PMC article.
-
Therapeutic immunoglobulin A antibody for dysbiosis-related diseases.Int Immunol. 2021 Nov 25;33(12):787-790. doi: 10.1093/intimm/dxab066. Int Immunol. 2021. PMID: 34492105 Free PMC article. Review.
-
Application of Microbiome Management in Therapy for Clostridioides difficile Infections: From Fecal Microbiota Transplantation to Probiotics to Microbiota-Preserving Antimicrobial Agents.Pathogens. 2021 May 24;10(6):649. doi: 10.3390/pathogens10060649. Pathogens. 2021. PMID: 34073695 Free PMC article. Review.
References
-
- Carter G. P., Chakravorty A., Pham Nguyen T. A., Mileto S., Schreiber F., Li L., et al. (2015). Defining the roles of TcdA and TcdB in localized gastrointestinal disease, systemic organ damage, and the host response during Clostridium difficile infections. MBio 6:e00551. 10.1128/mBio.00551-15 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

