Trichinella spiralis Thioredoxin Peroxidase 2 Regulates Protective Th2 Immune Response in Mice by Directly Inducing Alternatively Activated Macrophages
- PMID: 33072069
- PMCID: PMC7544948
- DOI: 10.3389/fimmu.2020.02015
Trichinella spiralis Thioredoxin Peroxidase 2 Regulates Protective Th2 Immune Response in Mice by Directly Inducing Alternatively Activated Macrophages
Abstract
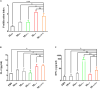
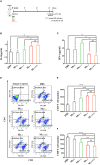
Trichinella infection can induce macrophages into the alternatively activated phenotype, which is primarily associated with the development of a polarized Th2 immune response. In the present study, we examined the immunomodulatory effect of T. spiralis thioredoxin peroxidase-2 (TsTPX2), a protein derived from T. spiralis ES products, in the regulation of Th2 response through direct activation of macrophages. The location of TsTPX2 was detected by immunohistochemistry and immunofluorescence analyses. The immune response in vivo induced by rTsTPX2 was characterized by analyzing the Th2 cytokines and Th1 cytokines in the peripheral blood. The rTsTPX2-activated macrophages (MrTsTPX2) were tested for polarization, their ability to evoke naïve CD4+ T cells, and resistance to the larval infection after adoptive transfer in BALB/c mice. The immunolocalization analysis showed TsTPX2 in cuticles and stichosome of T. spiralis ML. The immunostaining was detected in cuticles and stichosome of T. spiralis Ad3 and ML, as well as in tissue-dwellings around ML after the intestines and muscle tissues of infected mice were incubated with anti-rTsTPX2 antibody. Immunization of BALB/c mice with rTsTPX2 could induce a Th1-suppressing mixed immune response given the increased levels of Th2 cytokines (IL-4 and IL-10) production along with the decreased levels of Th1 cytokines (IFN-γ, IL-12, and TNF-α). In vitro studies showed that rTsTPX2 could directly drive RAW264.7 and peritoneal macrophages to the M2 phenotype. Moreover, MrTsTPX2 could promote CD4+ T cells polarized into Th2 type in vitro. Adoptive transfer of MrTsTPX2 into mice suppressed Th1 responses by enhancing Th2 responses and exhibited a 44.7% reduction in adult worm burden following challenge with T. spiralis infective larval, suggesting that the TsTPX2 is a potential vaccine candidate against trichinosis. Our study showed that TsTPX2 would be at least one of the molecules to switch macrophages into the M2 phenotype during T. spiralis infection, which provides a new therapeutic approach to various inflammatory disorders like allergies or autoimmune diseases.
Keywords: Th2 immune responses; Trichinella spiralis; alternative activation; macrophage; thioredoxin peroxidase-2.
Copyright © 2020 Jin, Zhang, Li, Qin, Liu, Ohiolei, Niu, Yan, Li, Jia, Song and Fu.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

