Macrophage TLR4 and PAR2 Signaling: Role in Regulating Vascular Inflammatory Injury and Repair
- PMID: 33072072
- PMCID: PMC7530636
- DOI: 10.3389/fimmu.2020.02091
Macrophage TLR4 and PAR2 Signaling: Role in Regulating Vascular Inflammatory Injury and Repair
Abstract
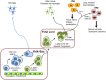
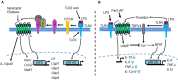
Macrophages play a central role in dictating the tissue response to infection and orchestrating subsequent repair of the damage. In this context, macrophages residing in the lungs continuously sense and discriminate among a wide range of insults to initiate the immune responses important to host-defense. Inflammatory tissue injury also leads to activation of proteases, and thereby the coagulation pathway, to optimize injury and repair post-infection. However, long-lasting inflammatory triggers from macrophages can impair the lung's ability to recover from severe injury, leading to increased lung vascular permeability and neutrophilic injury, hallmarks of Acute Lung Injury (ALI). In this review, we discuss the roles of toll-like receptor 4 (TLR4) and protease activating receptor 2 (PAR2) expressed on the macrophage cell-surface in regulating lung vascular inflammatory signaling.
Keywords: PAR2; TLR4; acute lung injury; alveolar macrophages; inflammation; macrophage; vascular permeability.
Copyright © 2020 Rayees, Rochford, Joshi, Joshi, Banerjee and Mehta.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

