Mir-331-3p Inhibits PRRSV-2 Replication and Lung Injury by Targeting PRRSV-2 ORF1b and Porcine TNF- α
- PMID: 33072088
- PMCID: PMC7544944
- DOI: 10.3389/fimmu.2020.547144
Mir-331-3p Inhibits PRRSV-2 Replication and Lung Injury by Targeting PRRSV-2 ORF1b and Porcine TNF- α
Abstract
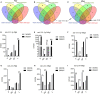
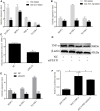
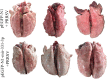
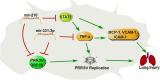
Porcine reproductive and respiratory syndrome (PRRS) caused by a single-stranded RNA virus (PRRSV) is a highly infectious respiratory disease and leads to huge economic losses to the swine industry worldwide. To investigate the role of miRNAs in the infection and lung injury induced by PRRSV, the differentially expressed miRNAs (DE-miRs) were isolated from PRRSV-2 infected/mock-infected PAMs of Meishan, Landrace, Pietrain, and Qingping pigs at 9, 36, and 60 hpi. Mir-331-3p was the only common DE-miR in each set of miRNA expression profile at 36 hpi. Mir-210 was one of 7 common DE-miRs between PRRSV infected and mock-infected PAMs of Meishan, Pietrain, and Qingping pigs at 60 hpi. Mir-331-3p/mir-210 could target PRRSV-2 ORF1b, bind and downregulate porcine TNF-α/STAT1 expression, and inhibit PRRSV-2 replication, respectively. Furthermore, STAT1 and TNF-α could mediate the transcriptional activation of MCP-1, VCAM-1, and ICAM-1. STAT1 could also upregulate the expression of TNF-α by binding to its promoter region. In vivo, pEGFP-N1-mir-331-3p could significantly reduce viral replication and pathological changes in PRRSV-2 infected piglets. Taken together, Mir-331-3p/mir-210 have significant roles in the infection and lung injury caused by PRRSV-2, and they may be promising therapeutic targets for PRRS and lung injury/inflammation.
Keywords: ORF1b; PRRSV; STAT1; TNF-α; lung injury; miR-210; miR-331-3p.
Copyright © 2020 You, Qu, Zhang, Huang, Gao, Huang, Luo, Liu, Liu and Xu.
Figures




Similar articles
-
miRNA let-7 family regulated by NEAT1 and ARID3A/NF-κB inhibits PRRSV-2 replication in vitro and in vivo.PLoS Pathog. 2022 Oct 10;18(10):e1010820. doi: 10.1371/journal.ppat.1010820. eCollection 2022 Oct. PLoS Pathog. 2022. PMID: 36215225 Free PMC article.
-
Downregulation of miR-296-3p by highly pathogenic porcine reproductive and respiratory syndrome virus activates the IRF1/TNF-α signaling axis in porcine alveolar macrophages.Arch Virol. 2021 Feb;166(2):511-519. doi: 10.1007/s00705-020-04921-y. Epub 2021 Jan 4. Arch Virol. 2021. PMID: 33394172
-
MiR-320 inhibits PRRSV replication by targeting PRRSV ORF6 and porcine CEBPB.Vet Res. 2024 May 15;55(1):61. doi: 10.1186/s13567-024-01309-7. Vet Res. 2024. PMID: 38750508 Free PMC article.
-
New perspective of host microRNAs in the control of PRRSV infection.Vet Microbiol. 2017 Sep;209:48-56. doi: 10.1016/j.vetmic.2017.01.004. Epub 2017 Jan 9. Vet Microbiol. 2017. PMID: 28161213 Review.
-
Host microRNAs as regulators of porcine reproductive and respiratory syndrome virus infection.Virology. 2025 Feb;603:110361. doi: 10.1016/j.virol.2024.110361. Epub 2024 Dec 21. Virology. 2025. PMID: 39721195 Review.
Cited by
-
miRNA let-7 family regulated by NEAT1 and ARID3A/NF-κB inhibits PRRSV-2 replication in vitro and in vivo.PLoS Pathog. 2022 Oct 10;18(10):e1010820. doi: 10.1371/journal.ppat.1010820. eCollection 2022 Oct. PLoS Pathog. 2022. PMID: 36215225 Free PMC article.
-
Host Cells Actively Resist Porcine Reproductive and Respiratory Syndrome Virus Infection via the IRF8-MicroRNA-10a-SRP14 Regulatory Pathway.J Virol. 2022 Apr 13;96(7):e0000322. doi: 10.1128/jvi.00003-22. Epub 2022 Mar 16. J Virol. 2022. PMID: 35293774 Free PMC article.
-
Regulatory Non-Coding RNAs during Porcine Viral Infections: Potential Targets for Antiviral Therapy.Viruses. 2024 Jan 13;16(1):118. doi: 10.3390/v16010118. Viruses. 2024. PMID: 38257818 Free PMC article. Review.
-
The circRNA circSIAE Inhibits Replication of Coxsackie Virus B3 by Targeting miR-331-3p and Thousand and One Amino-Acid Kinase 2.Front Cell Infect Microbiol. 2022 Jan 24;11:779919. doi: 10.3389/fcimb.2021.779919. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35141166 Free PMC article.
-
Strategies and scheming: the war between PRRSV and host cells.Virol J. 2025 Jun 11;22(1):191. doi: 10.1186/s12985-025-02685-y. Virol J. 2025. PMID: 40500743 Free PMC article. Review.
References
-
- Terpstra C, Wensvoort G, Pol JMA. Experimental reproduction of porcine epidemic abortion and respiratory syndrome (mystery swine disease) by infection with lelystad virus - koch postulates fulfilled (reprinted from the veterinary quarterly, Vol 13, Pg 131-136, 1991). Irish Vet J. (1993) 46:69–72. 10.1080/01652176.1991.9694297 - DOI - PubMed
-
- Collins JE, Benfield DA, Christianson WT, Harris L, Hennings JC, Shaw DP, et al. . Isolation of swine infertility and respiratory syndrome virus (Isolate atcc vr-2332) in north-america and experimental reproduction of the disease in gnotobiotic pigs. J Vet Diagn Invest. (1992) 4:117–26. 10.1177/104063879200400201 - DOI - PubMed
-
- Charoenchanikran P, Kedkovid R, Sirisereewan C, Woonwong Y, Arunorat J, Sitthichareonchai P, et al. . Efficacy of fosteraa (R) PRRS modified live virus (MLV) vaccination strategy against a Thai highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) infection. Trop Anim Health Prod. (2016) 48:1351–9. 10.1007/s11250-016-1099-1 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

