Familial Mediterranean Fever and COVID-19: Friends or Foes?
- PMID: 33072117
- PMCID: PMC7530822
- DOI: 10.3389/fimmu.2020.574593
Familial Mediterranean Fever and COVID-19: Friends or Foes?
Abstract
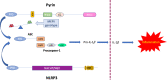
Familial Mediterranean Fever (FMF) and COVID-19 show a remarkable overlap of clinical symptoms and similar laboratory findings. Both are characterized by fever, abdominal/chest pain, elevation of C-reactive protein, and leukocytosis. In addition, colchicine and IL-1 inhibitors treatments that are effective in controlling inflammation in FMF patients have recently been proposed for off-label use in COVID-19 patients. Thus, FMF may resemble a milder recapitulation of the cytokine storm that is a hallmark of COVID-19 patients progressing to severe disease. We analyzed the sequence of the MEFV-encoded Pyrin protein - whose mutations cause FMF- in mammals, bats and pangolin. Intriguingly, although Pyrin is extremely conserved in species that are considered either a reservoir or intermediate hosts for SARS-CoV-2, some of the most common FMF-causing variants in humans are present as wildtype residues in these species. We propose that in humans, Pyrin may have evolved to fight highly pathogenic infections.
Keywords: COVID-19; FMF disease; cytokine storm; innate immunity; pyrin.
Copyright © 2020 Stella, Lamkanfi and Portincasa.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

