Evolution of Care of Orbital Tumors with Radiation Therapy
- PMID: 33072488
- PMCID: PMC7561458
- DOI: 10.1055/s-0040-1713894
Evolution of Care of Orbital Tumors with Radiation Therapy
Abstract
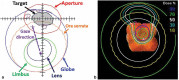
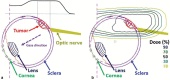
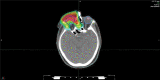
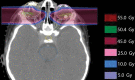
Orbital tumors are rare lesions comprising 0.1% of all tumors and less than 20% of all ocular diseases. These lesions in children and adults differ significantly in their incidence, tumor type, and treatment management. Although surgery and systemic therapies are commonly used in the management of these diseases, radiation therapy has become a widely used treatment for both benign and malignant tumors of the orbit. Radiotherapy is used as a definitive treatment to provide local control while avoiding morbidity associated with surgery for some tumors while it is used as an adjuvant treatment following surgical resection for others. For many tumors, radiation provides excellent tumor control with preservation of visual function. This article is dedicated for presenting the most common applications of orbital radiotherapy. A brief overview of the commonly available radiation therapy modalities is given. Dose constraint goals are reviewed and acute and long-term side effects are discussed. Orbital tumors covered in this article include optic glioma, ocular melanoma, retinoblastoma, orbital rhabdomyosarcoma, orbital lymphoma, and lacrimal gland tumors. Background information, indications for radiotherapy, and goals of treatment for each case example are described.
Keywords: IMRT; carbon-ion; carcinoma; glioma; lymphoma; melanoma; proton therapy; radiation; retinoblastoma; sarcoma.
Thieme. All rights reserved.
Conflict of interest statement
Conflict of Interest None declared.
Figures
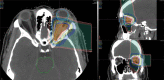
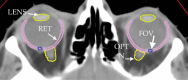
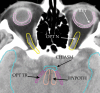
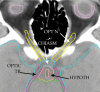

Similar articles
-
Fractionated, three-dimensional, planning-assisted proton-radiation therapy for orbital rhabdomyosarcoma: a novel technique.Int J Radiat Oncol Biol Phys. 2000 Jul 1;47(4):979-84. doi: 10.1016/s0360-3016(00)00545-9. Int J Radiat Oncol Biol Phys. 2000. PMID: 10863068
-
Survey of 1264 patients with orbital tumors and simulating lesions: The 2002 Montgomery Lecture, part 1.Ophthalmology. 2004 May;111(5):997-1008. doi: 10.1016/j.ophtha.2003.01.002. Ophthalmology. 2004. PMID: 15121380
-
Uveal melanomas. Conservation treatment.Hematol Oncol Clin North Am. 2001 Apr;15(2):389-402. doi: 10.1016/s0889-8588(05)70219-7. Hematol Oncol Clin North Am. 2001. PMID: 11370500 Review.
-
Radiation therapy: orbital tumors.Dev Ophthalmol. 2013;52:94-101. doi: 10.1159/000351084. Epub 2013 Aug 26. Dev Ophthalmol. 2013. PMID: 23989130 Review.
-
Importance of protocol target definition on the ability to spare normal tissue: an IMRT and 3D-CRT planning comparison for intraorbital tumors.Int J Radiat Oncol Biol Phys. 2005 Aug 1;62(5):1540-8. doi: 10.1016/j.ijrobp.2005.04.013. Int J Radiat Oncol Biol Phys. 2005. PMID: 16029816
Cited by
-
Ultra-low dose radiotherapy in the management of low-grade orbital lymphomas.Rep Pract Oncol Radiother. 2022 Jul 29;27(3):467-473. doi: 10.5603/RPOR.a2022.0044. eCollection 2022. Rep Pract Oncol Radiother. 2022. PMID: 36186691 Free PMC article.
-
Intensity-Modulated Radiation Therapy for Bilateral Choroidal Metastases Involving Macula and Optic Disc.Cureus. 2023 Oct 9;15(10):e46729. doi: 10.7759/cureus.46729. eCollection 2023 Oct. Cureus. 2023. PMID: 38022180 Free PMC article.
-
Ten years' blindness of the right eye: A rare presentation of an orbital melanoma.SAGE Open Med Case Rep. 2023 May 29;11:2050313X231173786. doi: 10.1177/2050313X231173786. eCollection 2023. SAGE Open Med Case Rep. 2023. PMID: 37284226 Free PMC article.
-
Extrapulmonary Small Cell Carcinoma Presenting as an Orbital Mass: A Case Report.Cureus. 2022 Jun 16;14(6):e26012. doi: 10.7759/cureus.26012. eCollection 2022 Jun. Cureus. 2022. PMID: 35734026 Free PMC article.
-
Mucosa-associated lymphoid tissue of nasopharynx: A case report and literature review.Radiol Case Rep. 2022 Jun 17;17(9):2991-2995. doi: 10.1016/j.radcr.2022.05.087. eCollection 2022 Sep. Radiol Case Rep. 2022. PMID: 35755107 Free PMC article.
References
-
- MacDonald S M, DeLaney T F, Loeffler J S. Proton beam radiation therapy. Cancer Invest. 2006;24(02):199–208. - PubMed
-
- Trofimov A, Bortfeld T. Optimization of beam parameters and treatment planning for intensity modulated proton therapy. Technol Cancer Res Treat. 2003;2(05):437–444. - PubMed
-
- MacDonald S M, Trofimov A, Safai S. Proton radiotherapy for pediatric central nervous system germ cell tumors: early clinical outcomes. Int J Radiat Oncol Biol Phys. 2011;79(01):121–129. - PubMed

