Angiotensin-converting enzyme 2 (ACE2): SARS-CoV-2 receptor and RAS modulator
- PMID: 33072500
- PMCID: PMC7553008
- DOI: 10.1016/j.apsb.2020.10.006
Angiotensin-converting enzyme 2 (ACE2): SARS-CoV-2 receptor and RAS modulator
Abstract
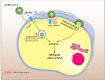
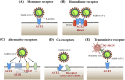
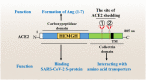
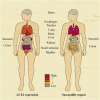
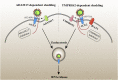
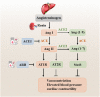
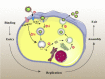
The coronavirus disease 2019 (COVID-19) outbreak is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Angiotensin-converting enzyme 2 (ACE2) was rapidly identified as the critical functional receptor for SARS-CoV-2. ACE2 is well-known as a counter-regulator of the renin-angiotensin system (RAS) and plays a key role in the cardiovascular system. Given that ACE2 functions as both a SARS-CoV-2 receptor and a RAS modulator, the treatment for COVID-19 presents a dilemma of how to limit virus entry but protect ACE2 physiological functions. Thus, an in-depth summary of the recent progress of ACE2 research and its relationship to the virus is urgently needed to provide possible solution to the dilemma. Here, we summarize the complexity and interplay between the coronavirus, ACE2 and RAS (including anti-RAS drugs). We propose five novel working modes for functional receptor for SARS-CoV-2 infection and the routes of ACE2-mediated virus entering host cells, as well as its regulatory mechanism. For the controversy of anti-RAS drugs application, we also give theoretical analysis and discussed for drug application. These will contribute to a deeper understanding of the complex mechanisms of underlying the relationship between the virus and ACE2, and provide guidance for virus intervention strategies.
Keywords: Angiotensin-converting enzyme 2; Anti-RAS drug; COVID-19; Drug target; Modulator; Receptor; Renin-angiotensin system; SARS-CoV-2.
© 2021 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures

References
-
- World Health Organization . 16 May 2020. COVID-2019 situation reports.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio... Available from:
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

