Impact of Age on the Efficacy of Immune Checkpoint Inhibitor-Based Combination Therapy for Non-small-Cell Lung Cancer: A Systematic Review and Meta-Analysis
- PMID: 33072551
- PMCID: PMC7538697
- DOI: 10.3389/fonc.2020.01671
Impact of Age on the Efficacy of Immune Checkpoint Inhibitor-Based Combination Therapy for Non-small-Cell Lung Cancer: A Systematic Review and Meta-Analysis
Abstract
Background: Despite the acknowledged benefits of immune checkpoint inhibitor (ICI)-based combination therapy (either with other checkpoint inhibitors, chemotherapy, targeted therapy, or radiotherapy), little is known about the impact of age on the efficacy of ICI -based combination therapy in non-small-cell lung cancer (NSCLC) patients. We conducted a systematic review and meta-analysis to investigate the differences in the benefits of ICI-based combination therapy for NSCLC by age (cut-off age, 65 years). Methods: We systematically searched randomized controlled trials (RCTs) of ICI plus other therapies including other ICIs, chemotherapies, targeted therapies, or radiotherapies, in the PubMed, Embase, and Cochrane databases with available hazard ratios (HRs) and 95% confidence intervals (CIs) for death and disease progression according to patient age. The search deadline was May 25, 2020. First, we calculated the pooled HRs of younger and older patients based on the HRs from each trial. Second, we assessed the pooled ratio of HRs reported in older patients to the HRs reported in younger patients for progression or death by the random-effects model. An estimated pooled HR ratio was lower than 1 indicating a better effect in older patients and higher than 1 indicating a better effect in younger patients. Results: A total of 10 eligible RCTs were included in our meta-analysis. The pooled HR for overall survival (OS) comparing ICI combined with other therapies to non-ICI regimens was 0.67 (95%CI 0.58-0.78) for younger patients and 0.79 (95%CI 0.70-0.90) for older patients. The pooled HRs ratio for OS reported in older patients compared to younger patients was 1.16 (95%CI 0.99-1.34), indicating no statistically significant difference between younger and older patients. Consistent with the findings related to OS, the analysis also demonstrated that ICI-based immunotherapy could significantly prolong progression-free survival (PFS) in younger and older patients (HR = 0.55; 95% CI 0.47-0.66, and HR = 0.64; 95% CI 0.57-0.71). The same results could also be observed in the pooled HRs ratio for PFS (HR = 1.15, 95%CI 0.91-1.46) indicating comparable efficacy of ICI-based combination therapy in younger and older patients with NSCLC. Conclusion: ICI-based combination therapy vs. non-ICI treatment had comparable efficacy in younger and older NSCLC patients with a cut-off age of 65 years.
Keywords: age; combination; immune checkpoint inhibitor; meta-analysis; non-small-cell lung cancer.
Copyright © 2020 Yan, Tian, Wu and Han.
Figures
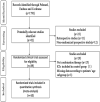

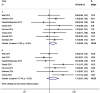
References
-
- West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. . Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2019) 20:924-37. 10.1016/S1470-2045(19)30167-6 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

