Plasma AR Copy Number Changes and Outcome to Abiraterone and Enzalutamide
- PMID: 33072600
- PMCID: PMC7542981
- DOI: 10.3389/fonc.2020.567809
Plasma AR Copy Number Changes and Outcome to Abiraterone and Enzalutamide
Abstract
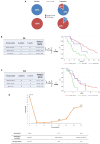
Introduction: Plasma androgen receptor (AR) copy number (CN) status identifies castration-resistant prostate cancer (CRPC) patients with worse outcome on abiraterone/enzalutamide. However, the impact of AR CN changes on clinical outcome in CRPC is unknown. Materials and Methods: Plasma samples from 73 patients treated with abiraterone or enzalutamide were collected at baseline and at the time of progression disease (PD). Droplet digital polymerase chain reaction was used to assess AR CN status. Results: We showed that 11 patients (15.1%) changed AR CN status from baseline to PD (9 patients from normal to gain, 2 from gain to normal). Patients changing AR CN status from normal at baseline to gain at PD had intermediate median overall survival (OS) of 20.5 months (95% CI = 8.0-44.2) between those who remained AR CN normal from baseline to PD (27.3 months [95% CI = 21.9-34.4]) and those who remained AR CN gain from baseline to PD (9.1 months [95% CI = 3.8-14.5], p < 0.0001). Patients changing AR CN from normal at baseline to gain at PD had a median progression-free survival (PFS) of 9.2 months (95% CI = 2.0-14.7), patients who remained AR CN normal had a median PFS of 9.1 months (95% CI = 7.2-10.1), and patients who remained AR CN gain had a median PFS of 5.4 (95% CI = 3.6-6.5, p = 0.0005). Both OS and PFS were not significantly different between patients with AR CN that changes from normal to gain and patients with stable AR CN normal. Conclusions: We showed that CRPC patients changing AR CN status from baseline to progression time point had intermediate OS and we suggested that AR CN evaluation at baseline could be the most informative for clinical outcome of CRPC patients treated with abiraterone or enzalutamide. Larger prospective studies are warranted.
Keywords: abiraterone acetate; androgen receptor; cell free DNA; clinical outcome; copy number changes; enzalutamide.
Copyright © 2020 Gurioli, Conteduca, Lolli, Schepisi, Gargiulo, Altavilla, Casadei, Scarpi and De Giorgi.
Figures
Similar articles
-
Prognostic and Therapeutic Implications of Circulating Androgen Receptor Gene Copy Number in Prostate Cancer Patients Using Droplet Digital Polymerase Chain Reaction.Clin Genitourin Cancer. 2018 Jun;16(3):197-205.e5. doi: 10.1016/j.clgc.2017.12.008. Epub 2017 Dec 29. Clin Genitourin Cancer. 2018. PMID: 29366632
-
Longitudinal assessment of plasma androgen receptor copy number predicts overall survival in subsequent treatment lines in castration-resistant prostate cancer: analysis from a prospective trial.ESMO Open. 2023 Dec;8(6):102036. doi: 10.1016/j.esmoop.2023.102036. Epub 2023 Oct 20. ESMO Open. 2023. PMID: 37866028 Free PMC article.
-
Baseline Plasma Tumor DNA (ctDNA) Correlates with PSA Kinetics in Metastatic Castration-Resistant Prostate Cancer (mCRPC) Treated with Abiraterone or Enzalutamide.Cancers (Basel). 2022 Apr 29;14(9):2219. doi: 10.3390/cancers14092219. Cancers (Basel). 2022. PMID: 35565349 Free PMC article.
-
Prognostic Value of Androgen Receptor Splice Variant 7 in the Treatment of Castration-resistant Prostate Cancer with Next generation Androgen Receptor Signal Inhibition: A Systematic Review and Meta-analysis.Eur Urol Focus. 2018 Jul;4(4):529-539. doi: 10.1016/j.euf.2017.01.004. Epub 2017 Jan 23. Eur Urol Focus. 2018. PMID: 28753843
-
AR Copy Number and AR Signaling-directed Therapies in Castrationresistant Prostate Cancer.Curr Cancer Drug Targets. 2018;18(9):869-876. doi: 10.2174/1568009617666171122145852. Curr Cancer Drug Targets. 2018. PMID: 29173173 Review.
Cited by
-
Changing metastatic patterns associate with dynamics of circulating tumor DNA in metastatic castration-resistant prostate cancer.Oncologist. 2025 May 8;30(5):oyaf107. doi: 10.1093/oncolo/oyaf107. Oncologist. 2025. PMID: 40377440 Free PMC article.
-
Liquid Biopsy Biomarkers in Metastatic Castration-Resistant Prostate Cancer Treated with Second-Generation Antiandrogens: Ready for Clinical Practice? A Systematic Review.Cancers (Basel). 2025 Jul 27;17(15):2482. doi: 10.3390/cancers17152482. Cancers (Basel). 2025. PMID: 40805181 Free PMC article. Review.
-
Systems-level liquid biopsy in advanced prostate cancer.Endocr Relat Cancer. 2025 Jan 17;32(3):e240274. doi: 10.1530/ERC-24-0274. Print 2025 Mar 1. Endocr Relat Cancer. 2025. PMID: 39752185 Review.
-
Current and Emerging Applications of Droplet Digital PCR in Oncology: An Updated Review.Mol Diagn Ther. 2022 Jan;26(1):61-87. doi: 10.1007/s40291-021-00562-2. Epub 2021 Nov 13. Mol Diagn Ther. 2022. PMID: 34773243 Review.
-
Predicting response to enzalutamide and abiraterone in metastatic prostate cancer using whole-omics machine learning.Nat Commun. 2023 Apr 8;14(1):1968. doi: 10.1038/s41467-023-37647-x. Nat Commun. 2023. PMID: 37031196 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

