Emerging Roles of SRSF3 as a Therapeutic Target for Cancer
- PMID: 33072610
- PMCID: PMC7544984
- DOI: 10.3389/fonc.2020.577636
Emerging Roles of SRSF3 as a Therapeutic Target for Cancer
Abstract
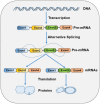
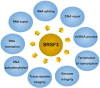
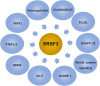
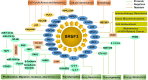
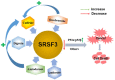
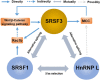
Ser/Arg-rich (SR) proteins are RNA-binding proteins known as constitutive and alternative splicing (AS) regulators that regulate multiple aspects of the gene expression program. Ser/Arg-rich splicing factor 3 (SRSF3) is the smallest member of the SR protein family, and its level is controlled by multiple factors and involves complex mechanisms in eukaryote cells, whereas the aberrant expression of SRSF3 is associated with many human diseases, including cancer. Here, we review state-of-the-art research on SRSF3 in terms of its function, expression, and misregulation in human cancers. We emphasize the negative consequences of the overexpression of the SRSF3 oncogene in cancers, the pathways underlying SRSF3-mediated transformation, and implications of potential anticancer drugs by downregulation of SRSF3 expression for cancer therapy. Cumulative research on SRSF3 provides critical insight into its essential part in maintaining cellular processes, offering potential new targets for anti-cancer therapy.
Keywords: RNA splicing; SRSF3; alternative splicing; cancer; oncogene.
Copyright © 2020 Zhou, Gong, Lin, Wang, Li, Wang, Ding and Li.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

