Control of Immediate Early Gene Expression for Human Cytomegalovirus Reactivation
- PMID: 33072616
- PMCID: PMC7533536
- DOI: 10.3389/fcimb.2020.00476
Control of Immediate Early Gene Expression for Human Cytomegalovirus Reactivation
Abstract
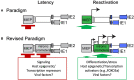
Human cytomegalovirus (HCMV) is a beta herpesvirus that persists for life in the majority of the world's population. The persistence of HCMV in the human population is due to the exquisite ability of herpesviruses to establish a latent infection that evades elimination by the host immune response. How the virus moves into and out of the latent state has been an intense area of research focus and debate. The prevailing paradigm is that the major immediate early promoter (MIEP), which drives robust expression of the major immediate early (MIE) transactivators, is epigenetically silenced during the establishment of latency, and must be reactivated for the virus to exit latency and re-enter productive replication. While it is clear that the MIEP is silenced by the association of repressive chromatin remodeling factors and histone marks, the mechanisms by which HCMV de-represses MIE gene expression for reactivation are less well understood. We have identified alternative promoter elements within the MIE locus that drive a second or delayed phase of MIE gene expression during productive infection. In the context of reactivation in THP-1 macrophages and primary CD34+ human progenitor cells, MIE transcripts are predominantly derived from initiation at these alternative promoters. Here we review the mechanisms by which alternative viral promoters might tailor the control of viral gene expression and the corresponding pattern of infection to specific cell types. Alternative promoter control of the HCMV MIE locus increases versatility in the system and allows the virus to tightly repress viral gene expression for latency but retain the ability to sense and respond to cell type-specific host cues for reactivation of replication.
Keywords: alternative promoter usage; cytomegalovirus; herpesvirus; latency; major immediate early (MIE) promoter; reactivation.
Copyright © 2020 Collins-McMillen, Kamil, Moorman and Goodrum.
Figures
Similar articles
-
Human cytomegalovirus major immediate early transcripts arise predominantly from the canonical major immediate early promoter in reactivating progenitor-derived dendritic cells.J Gen Virol. 2020 Jun;101(6):635-644. doi: 10.1099/jgv.0.001419. J Gen Virol. 2020. PMID: 32375946 Free PMC article.
-
Alternative promoters drive human cytomegalovirus reactivation from latency.Proc Natl Acad Sci U S A. 2019 Aug 27;116(35):17492-17497. doi: 10.1073/pnas.1900783116. Epub 2019 Aug 13. Proc Natl Acad Sci U S A. 2019. PMID: 31409717 Free PMC article.
-
Repression of the major immediate early promoter of human cytomegalovirus allows transcription from an alternate promoter.J Gen Virol. 2023 Sep;104(9). doi: 10.1099/jgv.0.001894. J Gen Virol. 2023. PMID: 37702591
-
Chromatin-mediated regulation of cytomegalovirus gene expression.Virus Res. 2011 May;157(2):134-43. doi: 10.1016/j.virusres.2010.09.019. Epub 2010 Sep 25. Virus Res. 2011. PMID: 20875471 Free PMC article. Review.
-
Chromatin structure regulates human cytomegalovirus gene expression during latency, reactivation and lytic infection.Biochim Biophys Acta. 2010 Mar-Apr;1799(3-4):286-95. doi: 10.1016/j.bbagrm.2009.08.001. Epub 2009 Aug 12. Biochim Biophys Acta. 2010. PMID: 19682613 Review.
Cited by
-
SOX2 downregulation of PML increases HCMV gene expression and growth of glioma cells.PLoS Pathog. 2023 Apr 14;19(4):e1011316. doi: 10.1371/journal.ppat.1011316. eCollection 2023 Apr. PLoS Pathog. 2023. PMID: 37058447 Free PMC article.
-
cGAS-STING-TBK1 Signaling Promotes Valproic Acid-Responsive Human Cytomegalovirus Immediate-Early Transcription during Infection of Incompletely Differentiated Myeloid Cells.Viruses. 2024 May 30;16(6):877. doi: 10.3390/v16060877. Viruses. 2024. PMID: 38932169 Free PMC article.
-
Does the Zinc Finger Antiviral Protein (ZAP) Shape the Evolution of Herpesvirus Genomes?Viruses. 2021 Sep 17;13(9):1857. doi: 10.3390/v13091857. Viruses. 2021. PMID: 34578438 Free PMC article. Review.
-
Cell type differences in human cytomegalovirus transcription and epigenetic regulation with insights into major immediate-early enhancer-promoter control.PLoS Pathog. 2025 Aug 4;21(8):e1013374. doi: 10.1371/journal.ppat.1013374. eCollection 2025 Aug. PLoS Pathog. 2025. PMID: 40758707 Free PMC article.
-
Lessons from Acquired Natural Immunity and Clinical Trials to Inform Next-Generation Human Cytomegalovirus Vaccine Development.Annu Rev Virol. 2022 Sep 29;9(1):491-520. doi: 10.1146/annurev-virology-100220-010653. Epub 2022 Jun 15. Annu Rev Virol. 2022. PMID: 35704747 Free PMC article. Review.

