Transcatheter Closure of Patent Ductus Arteriosus in Infants With Weight Under 1,500 Grams
- PMID: 33072674
- PMCID: PMC7536298
- DOI: 10.3389/fped.2020.558256
Transcatheter Closure of Patent Ductus Arteriosus in Infants With Weight Under 1,500 Grams
Abstract
Persistent patent ductus arteriosus (PDA) is very common in preterm infants, especially in extremely preterm infants. Despite significant advances in management of these vulnerable infants, there has been no consensus on management of PDA-when should we treat, who should we treat, how should we treat and in fact there is no agreement on how we should define a hemodynamically significant PDA. Medical management with non-steroidal anti-inflammatory drugs (NSAIDs) remains the first line of therapy with moderate success rate in closing the PDA. Paracetamol has been reported to be a safe and equally effective medical therapy for closure of PDA. However, additional studies on its long-term safety and efficacy in extremely low birth weight infants are needed before paracetamol can be recommended as standard treatment for a PDA in preterm infants. Surgical ligation of PDA is not without an increased risk of mortality and co-morbidities. Recently, there has been a significant interest in percutaneous transcatheter closure of PDA in preterm infants, including extremely low birth weight infants. Transcatheter PDA closure in preterm ELBW infants is technically feasible with high PDA occlusion success rates and acceptable complication rates as compared to surgical ligation. Many centers have reported promising early- and mid-term follow-up results. However, they need to be further tested in the prospective well-designed studies and randomized controlled trials comparing the results and outcomes of this technique with current treatment strategies including medical treatment before they can be used as the new standard of care for PDA closure in extremely low birth weight infants.
Keywords: extremely preterm infants; patent ductus arteriosus; patent ductus arteriosus (PDA); percutaneous closure; prematurity; transcatheter closure.
Copyright © 2020 Fraisse, Bautista-Rodriguez, Burmester, Lane and Singh.
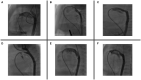
Figures



References
-
- Garland J, Buck R, Weinberg M. Pulmonary hemorrhage risk in infants with a clinically diagnosed patent ductus arteriosus: a retrospective cohort study. Pediatrics. (1994) 94:719–23. - PubMed
-
- Marshall DD, Kotelchuck M, Young TE, Bose CL, Kruyer L, O'Shea TM. Risk factors for chronic lung disease in the surfactant era: a North Carolina population-based study of very low birth weight infants. North Carolina Neonatologists Association. Pediatrics. (1999) 104:1345–50. 10.1542/peds.104.6.1345 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

