Evaluation of the Cost of Survivorship Care After Allogeneic Hematopoeitic Stem Cell Transplantation-An Analysis of 2 German Transplantation Centers
- PMID: 33072711
- PMCID: PMC7544947
- DOI: 10.3389/fpubh.2020.572470
Evaluation of the Cost of Survivorship Care After Allogeneic Hematopoeitic Stem Cell Transplantation-An Analysis of 2 German Transplantation Centers
Abstract
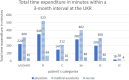
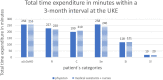
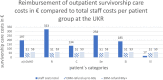
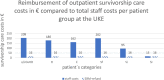
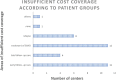
The aim of the presented study was to analyze the care expenditure for outpatients after allogeneic hematopoietic stem cell transplantation (alloHSCT) done in accordance with the national, European guidelines and the German Social Law. We performed an analysis of the National and European survivorship care guidelines and in parallel recorded the time expenditure and staff costs separated according to different occupational groups involved in outpatient care at two German transplantation centers [University Hospital Regensburg (UKR) and University Hospital Hamburg-Eppendorf (UKE)]. In addition, we performed a comparison of real costs vs. reimbursed costs according to the standard rating benchmark catalog (EBM), which was supplemented by a survey of German transplantation centers. The results showed that the staff costs are only covered by the EBM for patients without complications during long-term follow-up care-notably, this accounts for 15% of alloHSCT patients. Staff costs for patients requiring treatment of graft-vs.-host disease or relapse of the malignant underlying malignancy exceed to the factor 6.5 (UKR) to 12 (UKE) of the EBM revenue, caused both by the increased duration and frequency of the outpatient visits. As a result of the survey at German transplant centers, 15 out of 18 responding centers reported a lack of cost coverage for follow-up care. Two/15 centers reported that survivorship care is limited to a restricted time, independent of patient's needs, due to a lack of cost reimbursement. The results show that alloHSCT survivorship care of patients requires significant staff resources, which are not covered by the current version of the German EBM catalog. New approaches to finance labor intensive after care of transplant patients are required.
Keywords: Graft vs. Host disease; GvHD; costs; haematopoietic stem cell transplant; re-imbursement; survivorship care model.
Copyright © 2020 Wolff, Bardak, Edinger, Klinger-Schindler, Holler, Lawitschka, Schoemans, Herr, Kröger and Ayuk Ayuketang.
Figures
References
-
- Rizzo JD, Wingard JR, Tichelli A, Lee SJ, Van Lint MT, Burns LJ, et al. . Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, Center for International Blood and Marrow Transplant Research, and the American Society for Blood and Marrow Transplantation (EBMT/CIBMTR/ASBMT). Bone Marrow Transpl. (2006) 37:249–61. 10.1038/sj.bmt.1705243 - DOI - PubMed
-
- Sun CL, Kersey JH, Francisco L, Armenian SH, Baker KS, Weisdorf DJ, et al. . Burden of morbidity in 10+ year survivors of hematopoietic cell transplantation: report from the bone marrow transplantation survivor study. Biol Blood Marrow Transpl. (2013) 19:1073–80. 10.1016/j.bbmt.2013.04.002 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

