Mesenchymal Stromal Cell-Produced Components of Extracellular Matrix Potentiate Multipotent Stem Cell Response to Differentiation Stimuli
- PMID: 33072743
- PMCID: PMC7536557
- DOI: 10.3389/fcell.2020.555378
Mesenchymal Stromal Cell-Produced Components of Extracellular Matrix Potentiate Multipotent Stem Cell Response to Differentiation Stimuli
Abstract
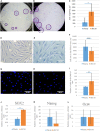
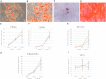
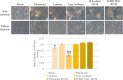
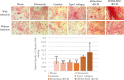
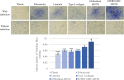
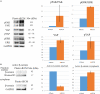
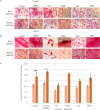
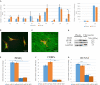
Extracellular matrix (ECM) provides both structural support and dynamic microenvironment for cells regulating their behavior and fate. As a critical component of stem cell niche ECM maintains stem cells and activates their proliferation and differentiation under specific stimuli. Mesenchymal stem/stromal cells (MSCs) regulate tissue-specific stem cell functions locating in their immediate microenvironment and producing various bioactive factors, including ECM components. We evaluated the ability of MSC-produced ECM to restore stem and progenitor cell microenvironment in vitro and analyzed the possible mechanisms of its effects. Human MSC cell sheets were decellularized by different agents (detergents, enzymes, and apoptosis inductors) to select the optimized combination (CHAPS and DNAse I) based on the conservation of decellularized ECM (dECM) structure and effectiveness of DNA removal. Prepared dECM was non-immunogenic, supported MSC proliferation and formation of larger colonies in colony-forming unit-assay. Decellularized ECM effectively promoted MSC trilineage differentiation (adipogenic, osteogenic, and chondrogenic) compared to plastic or plastic covered by selected ECM components (collagen, fibronectin, laminin). Interestingly, dECM produced by human fibroblasts could not enhance MSC differentiation like MSC-produced dECM, indicating cell-specific functionality of dECM. We demonstrated the significant integrin contribution in dECM-cell interaction by blocking the stimulatory effects of dECM with RGD peptide and suggested the involvement of key intracellular signaling pathways activation (pERK/ERK and pFAK/FAK axes, pYAP/YAP and beta-catenin) in the observed processes based on the results of inhibitory analysis. Taken together, we suppose that MSC-produced dECM may mimic stem cell niche components in vitro and maintain multipotent progenitor cells to insure their effective response to external differentiating stimuli upon activation. The obtained data provide more insights into the possible role of MSC-produced ECM in stem and progenitor cell regulation within their niches. Our results are also useful for the developing of dECM-based cell-free products for regenerative medicine.
Keywords: decellularization; extracellular matrix; mesenchymal stromal cells; progenitor; stem cell niche.
Copyright © 2020 Novoseletskaya, Grigorieva, Nimiritsky, Basalova, Eremichev, Milovskaya, Kulebyakin, Kulebyakina, Rodionov, Omelyanenko and Efimenko.
Figures


Similar articles
-
Tailored environments for directed mesenchymal stromal cell proliferation and differentiation using decellularized extracellular matrices in conjunction with substrate modulus.Acta Biomater. 2024 Oct 1;187:110-122. doi: 10.1016/j.actbio.2024.08.022. Epub 2024 Aug 22. Acta Biomater. 2024. PMID: 39181177
-
Native and solubilized decellularized extracellular matrix: A critical assessment of their potential for improving the expansion of mesenchymal stem cells.Acta Biomater. 2017 Jun;55:1-12. doi: 10.1016/j.actbio.2017.04.014. Epub 2017 Apr 12. Acta Biomater. 2017. PMID: 28412553 Review.
-
Bioactivity of human adult stem cells and functional relevance of stem cell-derived extracellular matrix in chondrogenesis.Stem Cell Res Ther. 2023 Jun 14;14(1):160. doi: 10.1186/s13287-023-03392-7. Stem Cell Res Ther. 2023. PMID: 37316923 Free PMC article.
-
Age associated communication between cells and matrix: a potential impact on stem cell-based tissue regeneration strategies.Organogenesis. 2014;10(3):289-98. doi: 10.4161/15476278.2014.970089. Organogenesis. 2014. PMID: 25482504 Free PMC article. Review.
-
Transferable Matrixes Produced from Decellularized Extracellular Matrix Promote Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells and Facilitate Scale-Up.ACS Biomater Sci Eng. 2018 May 14;4(5):1760-1769. doi: 10.1021/acsbiomaterials.7b00747. Epub 2018 Apr 16. ACS Biomater Sci Eng. 2018. PMID: 33445333
Cited by
-
Effects of mesenchymal stem cell on dopaminergic neurons, motor and memory functions in animal models of Parkinson's disease: a systematic review and meta-analysis.Neural Regen Res. 2024 Jul 1;19(7):1584-1592. doi: 10.4103/1673-5374.387976. Epub 2023 Nov 8. Neural Regen Res. 2024. PMID: 38051903 Free PMC article.
-
Advances in xenogeneic donor decellularized organs: A review on studies with sheep and porcine-derived heart valves.Bioeng Transl Med. 2024 Jul 3;9(6):e10695. doi: 10.1002/btm2.10695. eCollection 2024 Nov. Bioeng Transl Med. 2024. PMID: 39545084 Free PMC article. Review.
-
From Cells to Exosomes: a Review of Non-Surgical Biotherapeutic-Based Strategies for Liver Regeneration in the Face of End-Stage Diseases.Stem Cell Rev Rep. 2025 Aug;21(6):1669-1681. doi: 10.1007/s12015-025-10872-1. Epub 2025 May 24. Stem Cell Rev Rep. 2025. PMID: 40411652 Review.
-
Unlocking the Promise of Decellularized Pancreatic Tissue: A Novel Approach to Support Angiogenesis in Engineered Tissue.Bioengineering (Basel). 2024 Feb 14;11(2):183. doi: 10.3390/bioengineering11020183. Bioengineering (Basel). 2024. PMID: 38391669 Free PMC article.
-
Rejuvenation of Senescent Mesenchymal Stem Cells to Prevent Age-Related Changes in Synovial Joints.Cell Transplant. 2023 Jan-Dec;32:9636897231200065. doi: 10.1177/09636897231200065. Cell Transplant. 2023. PMID: 37766590 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

