Non-ciliary Roles of IFT Proteins in Cell Division and Polycystic Kidney Diseases
- PMID: 33072760
- PMCID: PMC7536321
- DOI: 10.3389/fcell.2020.578239
Non-ciliary Roles of IFT Proteins in Cell Division and Polycystic Kidney Diseases
Abstract
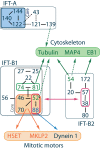
Cilia are small organelles present at the surface of most differentiated cells where they act as sensors for mechanical or biochemical stimuli. Cilia assembly and function require the Intraflagellar Transport (IFT) machinery, an intracellular transport system that functions in association with microtubules and motors. If IFT proteins have long been studied for their ciliary roles, recent evidences indicate that their functions are not restricted to the cilium. Indeed, IFT proteins are found outside the ciliary compartment where they are involved in a variety of cellular processes in association with non-ciliary motors. Recent works also provide evidence that non-ciliary roles of IFT proteins could be responsible for the development of ciliopathies related phenotypes including polycystic kidney diseases. In this review, we will discuss the interactions of IFT proteins with microtubules and motors as well as newly identified non-ciliary functions of IFT proteins, focusing on their roles in cell division. We will also discuss the potential contribution of non-ciliary IFT proteins functions to the etiology of kidney diseases.
Keywords: cell division; ciliopathies; intraflagellar transport; microtubule – associated proteins; molecular motor.
Copyright © 2020 Vitre, Guesdon and Delaval.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

