Hospitalized COVID-19 patients treated with convalescent plasma in a mid-size city in the Midwest
- PMID: 33072871
- PMCID: PMC7549340
- DOI: 10.1186/s41231-020-00068-9
Hospitalized COVID-19 patients treated with convalescent plasma in a mid-size city in the Midwest
Abstract
Background: SARS-CoV-2 and its associated disease, COVID-19, has infected over seven million people world-wide, including two million people in the United States. While many people recover from the virus uneventfully, a subset of patients will require hospital admission, some with intensive care needs including intubation, and mechanical ventilation. To date there is no cure and no vaccine is available. Passive immunotherapy by the transfusion of convalescent plasma donated by COVID-19 recovered patients might be an effective option to combat the virus, especially if used early in the course of disease. Here we report our experience of using convalescent plasma at a tertiary care center in a mid-size, midwestern city that did not experience an overwhelming patient surge.
Methods: Hospitalized COVID-19 patients categorized as having Severe or Life-Threatening disease according to the Mayo Clinic Emergency Access Protocol were screened, consented, and treated with convalescent plasma collected from local donors recovered from COVID-19 infection. Clinical data and outcomes were collected retrospectively.
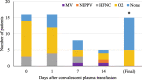
Results: 31 patients were treated, 16 severe patients and 15 life-threatened patients. Overall mortality was 27% (4/31) but only patients with life-threatening disease died. 94% of transfused patients with severe disease avoided escalation to ICU care and mechanical ventilation. 67% of patients with life-threatening disease were able to be extubated. Most transfused patients had a rapid decrease in their respiratory support requirements on or about day 7 following convalescent plasma transfusion.
Conclusion: Our results demonstrate that convalescent plasma is associated with reducing ventilatory requirements in patients with both severe and life-threatening disease, but appears to be most beneficial when administered early in the course of disease when patients meet the criteria for severe illness.
Keywords: COVID-19; Case series; Convalescent plasma; Midwest; SARS-CoV-2.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare they have no competing interests in the publication of this report.
Figures

Update of
-
Hospitalized COVID-19 patients treated with Convalescent Plasma in a mid-size city in the midwest.medRxiv [Preprint]. 2020 Jun 22:2020.06.19.20135830. doi: 10.1101/2020.06.19.20135830. medRxiv. 2020. Update in: Transl Med Commun. 2020;5(1):17. doi: 10.1186/s41231-020-00068-9. PMID: 32607514 Free PMC article. Updated. Preprint.
-
Hospitalized COVID-19 Patients treated with Convalescent Plasma in a Mid-size City in the Midwest.Res Sq [Preprint]. 2020 Jul 14:rs.3.rs-39447. doi: 10.21203/rs.3.rs-39447/v1. Res Sq. 2020. Update in: Transl Med Commun. 2020;5(1):17. doi: 10.1186/s41231-020-00068-9. PMID: 32702731 Free PMC article. Updated. Preprint.
-
Hospitalized COVID-19 Patients treated with Convalescent Plasma in a Mid-size City in the Midwest.Res Sq [Preprint]. 2020 Sep 17:rs.3.rs-54167. doi: 10.21203/rs.3.rs-54167/v2. Res Sq. 2020. Update in: Transl Med Commun. 2020;5(1):17. doi: 10.1186/s41231-020-00068-9. PMID: 32793897 Free PMC article. Updated. Preprint.
References
-
- https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-in.... Accessed 3 Apr 2020.
-
- Hess AS, Connor J, Roginski C, Hesse K, Weiss JW, Hartman W. Rapid development and refinement of a COVID-19 convalescent plasma donor recruitment and screening program. Abstract submitted to the American Association of Blood Banks Annual Meeting, Baltimore, Maryland; 2020, October 3-6.
LinkOut - more resources
Full Text Sources
Miscellaneous
